Abstract
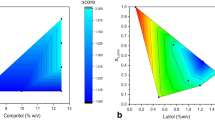
Nanosizing is frequently used as formulation approach to increase the bioavailability of poorly water-soluble drugs. However, standard size reduction processes can be relatively time-consuming. It was found that the modification of the physical properties of a starting material by means of spray-drying can be used to improve the effectiveness of a subsequently performed high pressure homogenization. Such a process belongs to the combinative particle size reduction methods and is also referred to as H 42 process. Based on previous studies, it was hypothesized that the improved efficiency was a result of reduced crystallinity of the modified drug. The present study was conducted in order to asses this hypothesis in a systematical manner by applying design of experiment (DoE) principles. Resveratrol was selected as model compound for this study. It was processed by both standard high pressure homogenization and by a combinative particle size reduction process (the H42 process). An optimized resveratrol/surfactant ratio for the spray-dried intermediate was identified by using the response-surface methodology. The optimization led to a nanosuspension with a mean particle size of 192 nm, which is much smaller than the mean particle size of 569 nm when standard high pressure homogenization was used. Both predominately crystalline and predominately amorphous solids resulted from the spray-drying process. In contrast to the initial hypothesis, the smallest particle sizes were achieved by processing predominately crystalline intermediate with high pressure homogenization.








Similar content being viewed by others
REFERENCES
Kesisoglou F, Panmai S, Wu Y. Nanosizing—oral formulation development and biopharmaceutical evaluation. Adv Drug Deliv Rev. 2007;59(7):631–44.
Kwade A. Wet comminution in stirred media mills—research and its practical application. Powder Technol. 1999;105(1–3):14–20.
Keck CM, Müller RH. Drug nanocrystals of poorly soluble drugs produced by high pressure homogenisation. Eur J Pharm Biopharm. 2006;62(1):3–16.
Merisko-Liversidge E, Liversidge GG. Nanosizing for oral and parenteral drug delivery: a perspective on formulating poorly-water soluble compounds using wet media milling technology. Adv Drug Deliv Rev. 2011;63(6):427–40.
Knieke C, Sommer M, Peukert W. Identifying the apparent and true grinding limit. Powder Technol. 2009;195(1):25–30.
Möschwitzer JP. Drug nanocrystals in the commercial pharmaceutical development process. Int J Pharm. 2013;453(1):142–56.
Salazar J, Müller RH, Möschwitzer JP. Performance comparison of two novel combinative particle-size-reduction technologies. J Pharm Sci. 2013;102(5):1636–49.
Möschwitzer J, Müller RH. New method for the effective production of ultrafine drug nanocrystals. J Nanosci Nanotechnol. 2006;6(9–10):3145–53.
Yu L. Amorphous pharmaceutical solids: preparation, characterization and stabilization. Adv Drug Deliv Rev. 2001;48(1):27–42.
Hancock BC, Zografi G. Characteristics and significance of the amorphous state in pharmaceutical systems. J Pharm Sci. 1997;86(1):1–12.
Huang Y, Luo X, You X, Xia Y, Song X, Yu L. The preparation and evaluation of water-soluble SKLB610 nanosuspensions with improved bioavailability. AAPS PharmSciTech. 2013;14(3):1236–43.
Bucher CG, Bourgund U. A fast and efficient response-surface approach for structural reliability problems. Struct Saf. 1990;7(1):57–66.
Singare DS, Marella S, Gowthamrajan K, Kulkarni GT, Vooturi R, Rao PS. Optimization of formulation and process variable of nanosuspension: an industrial perspective. Int J Pharm. 2010;402(1–2):213–20.
Verma S, Lan Y, Gokhale R, Burgess DJ. Quality by design approach to understand the process of nanosuspension preparation. Int J Pharm. 2009;377(1–2):185–98.
Jang MS, Cai EN, Udeani GO, Slowing KV, Thomas CF, Beecher CWW, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275(5297):218–20.
Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444(7117):337–42.
Amri A, Chaumeil JC, Sfar S, Charrueau C. Administration of resveratrol: what formulation solutions to bioavailability limitations? J Control Release. 2012;158(2):182–93.
Kobierski S, Ofori-Kwakye K, Müller RH, Keck CM. Resveratrol nanosuspensions for dermal application—production, characterization, and physical stability. Pharmazie. 2009;64(11):741–7.
Popova M, Szegedi A, Mavrodinova V, Tušar NN, Mihály J, Klébert S, et al. Preparation of resveratrol-loaded nanoporous silica materials with different structures. J Solid State Chem. 2014;219:37–42.
Walton D, Mumford C. The morphology of spray-dried particles: the effect of process variables upon the morphology of spray-dried particles. Chem Eng Res Des. 1999;77:442–60.
Salazar J, Müller RH, Möschwitzer JP. Application of the combinative particle size reduction technology H 42 to produce fast dissolving glibenclamide tablets. Eur J Pharm Sci. 2013;49(4):565–77.
Patterson JE, James MB, Forster AH, Lancaster RW, Butler JM, Rades T. The influence of thermal and mechanical preparative techniques on the amorphous state of four poorly soluble compounds. J Pharm Sci. 2005;94(9):1998–2012.
Stenger F, Mende S, Schwedes J, Peukert W. Nanomilling in stirred media mills. Chem Eng Sci. 2005;60:4557–65.
Torrado G, Fraile S, Torrado S, Torrado S. Process-induced crystallite size and dissolution changes elucidated by a variety of analytical methods. Int J Pharm. 1998;166:55–63.
ACKNOWLEDGMENTS
Tao Liu would like to thank China Scholarship Council (CSC) for the financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, T., Müller, R.H. & Möschwitzer, J.P. Systematical investigation of a combinative particle size reduction technology for production of resveratrol nanosuspensions. AAPS PharmSciTech 18, 1683–1691 (2017). https://doi.org/10.1208/s12249-016-0612-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-016-0612-1