Abstract
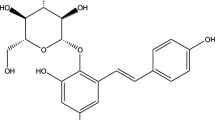
Huang-Lian-Jie-Du-Tang (HLJDT) is a classical recipe for relieving fever and toxicity for thousands of years in China. Geniposide is one of the main components in HLJDT. The present study was conducted in order to investigate the differences of absorption of geniposide after oral administration of geniposide alone and HLJDT in rats. Pharmacokinetic differences of geniposide following oral administrations of pure geniposide and HLJDT were investigated in vivo. The absorption of geniposide in pure compound and HLJDT was evaluated using intestinal perfusion and Caco-2 models. The in vivo and in vitro studies showed good relevance and consistent results. The co-occurring components in HLJDT were found to promote the absorption of geniposide from the pharmacokinetic study in vivo, intestinal perfusion, and Caco-2 model. Geniposide had better absorption in the duodenum and jejunum from the intestinal perfusion model, which was mainly absorbed by passive diffusion. Verapamil influenced the transportation of geniposide, while EDTA did not, demonstrating that geniposide might be the potential substance of P-glycoprotein in intestinal perfusion and Caco-2 models. The absorption of geniposide was studied systematically to guide the design of the oral dosage of geniposide and HLJDT in clinical therapy.




Similar content being viewed by others
References
Lu T, Song J, Huang F, Deng Y, Xie L, Wang G, et al. Comparative pharmacokinetics of baicalin after oral administration of pure baicalin, Radix scutellariae extract and Huang-Lian-Jie-Du-Tang to rats. J Ethnopharmacol. 2007;110(3):412–8. doi:10.1016/j.jep.2006.09.036.
Peng J, Guo N, Fan B, Yan H, Nie YL, Yu YH. Studies on HPLC fingerprint of Huanglian Jiedu decoction and quantitative analysis of index components. Chin J Exp Trad Med Form. 2011;10:67–70.
Z QW, Li YG. Berberine attenuates myocardial ischemia reperfusion injury by suppressing the activation of PI3K/AKT signaling. Exp Ther Med. 2016;11(3):978–84. doi:10.3892/etm.2016.3018.
Yang G, Li JQ, Bo JP, Wang B, Tian XR, Liu TZ, et al. Baicalin inhibits PDGF-induced proliferation and migration of airway smooth muscle cells. Int J Clin Exp Med. 2015;8(11):20532–9.
Chula S, Hang L, Yinying B, Jianning S, Shi R. The effects of notoginsenoside R(1) on the intestinal absorption of geniposide by the everted rat gut sac model. J Ethnopharmacol. 2012;142(1):136–43. doi:10.1016/j.jep.2012.04.027.
Kim YH, Jeong DW, Kim YC, Sohn DH, Park ES, Lee HS. Pharmacokinetics of baicalein, baicalin and wogonin after oral administration of a standardized extract of Scutellaria baicalensis, PF-2405 in rats. Arch Pharm Res. 2007;30(2):260–5.
Kotani A, Kojima S, Hakamata H, Kusu F. HPLC with electrochemical detection to examine the pharmacokinetics of baicalin and baicalein in rat plasma after oral administration of a Kampo medicine. Anal Biochem. 2006;350(1):99–104. doi:10.1016/j.ab.2005.11.007.
Pan L, Wang W, Shi F, Zhou J, Zhang M, Zhu H, et al. Exploratory pharmacokinetics of geniposide in rat model of cerebral ischemia orally administered with or without baicalin and/or berberine. Evid Based Complement Alternat Med. 2013;2013:349531. doi:10.1155/2013/349531.
Zeng MF, Pan LM, Zhu HX, Zhang QC, Guo LW. Comparative pharmacokinetics of baicalin in plasma after oral administration of Huang-Lian-Jie-Du-Tang or pure baicalin in MCAO and sham-operated rats. Fitoterapia. 2010;81(6):490–6. doi:10.1016/j.fitote.2010.01.004.
Zhang ZQ, Liua W, Zhuang L, Wang J, Zhang S. Comparative pharmacokinetics of baicalin, wogonoside, baicalein and wogonin in plasma after oral administration of pure baicalin, Radix Scutellariae and Scutellariae-Paeoniae couple extracts in normal and ulcerative colitis rats. Iran J Pharm Res. 2013;12(3):399–409.
Wang N, Feng Y, Tan HY, Cheung F, Hong M, Lao L, et al. Inhibition of eukaryotic elongation factor-2 confers to tumor suppression by a herbal formulation Huanglian-Jiedu decoction in human hepatocellular carcinoma. J Ethnopharmacol. 2015;164:309–18. doi:10.1016/j.jep.2015.02.025.
Ohta Y, Kongo-Nishimura M, Hayashi T, Kishikawa T. Effect of Oren-gedoku-to (Huanglian-Jie-Du-Tang) extract on disruption of hepatic antioxidant defense systems in rats treated with D-galactosamine. J Ethnopharmacol. 2004;94(2-3):323–9. doi:10.1016/j.jep.2004.06.004.
Xu J, Murakami Y, Matsumoto K, Tohda M, Watanabe H, Zhang S, et al. Protective effect of Oren-gedoku-to (Huang-Lian-Jie-Du-Tang) against impairment of learning and memory induced by transient cerebral ischemia in mice. J Ethnopharmacol. 2000;73(3):405–13.
Zhang Q, Bian H, Li Y, Guo L, Tang Y, Zhu H. Preconditioning with the traditional Chinese medicine Huang-Lian-Jie-Du-Tang initiates HIF-1alpha-dependent neuroprotection against cerebral ischemia in rats. J Ethnopharmacol. 2014;154(2):443–52. doi:10.1016/j.jep.2014.04.022.
Zou H, Long J, Zhang Q, Zhao H, Bian B, Wang Y, et al. Induced cortical neurogenesis after focal cerebral ischemia—three active components from Huang-Lian-Jie-Du decoction. J Ethnopharmacol. 2015. doi:10.1016/j.jep.2015.12.001.
Zheng WX, Cao XL, Wang F, Wang J, Ying TZ, Xiao W, et al. Baicalin inhibiting cerebral ischemia/hypoxia-induced neuronal apoptosis via MRTF-A-mediated transactivity. Eur J Pharmacol. 2015;767:201–10. doi:10.1016/j.ejphar.2015.10.027.
Kalapos-Kovacs B, Magda B, Jani M, Fekete Z, Szabo PT, Antal I, et al. Multiple ABC transporters efflux baicalin. Phytother Res. 2015;29(12):1987–90. doi:10.1002/ptr.5477.
Zhang Y, Cui YL, Gao LN, Jiang HL. Effects of beta-cyclodextrin on the intestinal absorption of berberine hydrochloride, a P-glycoprotein substrate. Int J Biol Macromol. 2013;59:363–71. doi:10.1016/j.ijbiomac.2013.04.074.
Zhang Y, Zhu HX, Guo LW. Intestinal absorption of berberine alone and in combinations by rats single pass intestinal perfusion in situ. Yao Xue Xue Bao. 2012;47(2):233–8.
Chen Z, Gong X, Lu Y, Du S, Yang Z, Bai J, et al. Enhancing effect of borneol and muscone on geniposide transport across the human nasal epithelial cell monolayer. PLoS One. 2014;9(7):e101414. doi:10.1371/journal.pone.0101414.
Zhu H, Qian Z, He F, Liu M, Pan L, Zhang Q, et al. Novel pharmacokinetic studies of the Chinese formula Huang-Lian-Jie-Du-Tang in MCAO rats. Phytomedicine. 2013;20(10):767–74. doi:10.1016/j.phymed.2012.11.012.
Zhu H, Qian Z, Li H, Guo L, Pan L, Zhang Q, et al. Integrated pharmacokinetics of major bioactive components in MCAO rats after oral administration of Huang-Lian-Jie-Du-Tang. J Ethnopharmacol. 2012;141(1):158–69. doi:10.1016/j.jep.2012.02.014.
Zhu HX, Zhang XL, Zeng MF, Guo LW, Pan LM. Correlation between in vivo pharmacodynamics and pharmacokinetics of berberine in Huanglian Jiedu decoction used for rats with cerebral ischemia. Chin Trad & Herb Drugs. 2012;03:546–51.
Wu YN, Luan LB. In situ rats single pass perfusion intestinal absorption of the effective components in Radix Angelicae Pubescentis. Yao Xue Xue Bao. 2008;43(1):102–7.
Levitt MD, Kneip JM, Levitt DG. Use of laminar flow and unstirred layer models to predict intestinal absorption in the rat. J Clin Invest. 1988;81(5):1365–9. doi:10.1172/jci113464.
Grassi M, Cadelli G. Theoretical considerations on the in vivo intestinal permeability determination by means of the single pass and recirculating techniques. Int J Pharm. 2001;229(1-2):95–105.
Salphati L, Childers K, Pan L, Tsutsui K, Takahashi L. Evaluation of a single-pass intestinal-perfusion method in rat for the prediction of absorption in man. J Pharm Pharmacol. 2001;53(7):1007–13.
Awortwe C, Fasinu PS, Rosenkranz B. Application of Caco-2 cell line in herb-drug interaction studies: current approaches and challenges. J Pharm Pharm Sci. 2014;17(1):1–19.
Chaw CS, Yazaki E, Evans DF. The effect of pH change on the gastric emptying of liquids measured by electrical impedance tomography and pH-sensitive radiotelemetry capsule. Int J Pharm. 2001;227(1-2):167–75.
Zhang Q, Ye YL, Yan YX, Zhang WP, Chu LS, Wei EQ, et al. Protective effects of Huanglian-Jiedu-Tang on chronic brain injury after focal cerebral ischemia in mice. J Zhejiang Univ (Med Sci). 2009;(01):75–80.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (Project Nos. 81573635; 30873450) and funded by the Natural Science Foundation of Jiangsu Province (Project Nos. BK 2012855; BY 2012036), the Priority Academic Program Development of Jiangsu Higher Education Institutions (ysxk-2010), the Six Talent Project in Jiangsu Province, the Administration of Traditional Chinese Medicine of Jiangsu Province (No. LZ13007), and the Innovation Research Team of Nanjing University of Chinese Medicine (2013).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Yu, D., Zhang, Y., Guo, L. et al. Study on the Absorption Mechanism of Geniposide in the Chinese Formula Huang-Lian-Jie-Du-Tang in Rats. AAPS PharmSciTech 18, 1382–1392 (2017). https://doi.org/10.1208/s12249-016-0610-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-016-0610-3