Abstract
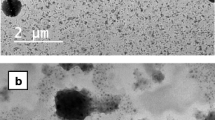
This research focuses on the fabrication and evaluation of solid lipid nanoparticles (SLNs) for improved ocular delivery of valacyclovir (VAC). Stearic acid and tristearin were selected as the lipid carrier while Poloxamer 188 and sodium taurocholate were used as surfactant and co-surfactant, respectively. The physiochemical properties of the optimized batch (SLN-6) fulfil the prerequisites needed for an ideal ocular formulation like submicron size (202.5 ± 2.56 nm), narrow PDI (0.252 ± 0.06), high zeta potential (−34.4 ± 3.04 mV) and good entrapment efficiency (58.82 ± 2.45%). The in vitro release study of SLN-6 exhibited a sustained release profile (>60% in 12 h). The ex vivo studies performed on excised cornea exhibited enhanced drug permeation of SLNs (22.17 ± 1.41 μg/cm2 h) in comparison to the drug solution (3.78 ± 1.34 μg/cm2 h). Apart, the corneal hydration studies, histopathology and Hen’s Egg Test Chorio Allantoic Membrane (HETCAM) assay, confirmed the non-irritancy of SLNs. The in vivo study confirmed improved ocular bioavailability of VAC from SLN-6 (AUC0–12: 856.47 ± 7.86 μg h/mL) in contrast to the drug solution (AUC0–12: 470.75 ± 8.91 μg h/mL). Hence, the overall studies suggested the potential of SLNs in efficient ocular delivery of a hydrophilic molecule like VAC.










Similar content being viewed by others
References
Hill GM, Ku ES, Dwarakanathan S. Herpes simplexkeratitis. Dis Mon. 2014;60:239–46.
Kaye S, Choudhary A. Herpes simplex keratitis. Prog Retin Eye Res. 2006;25:355–80.
Miserocchi E, Modorati G, Galli L, Rama P. Efficacy of valacyclovir vs acyclovir for the prevention of recurrent herpes simplex virus eye disease: a pilot study. Am J Ophthalmol. 2007;144:547–51.
Katragadda S, Gunda S, Hariharan S, Mitra AK. Ocular pharmacokinetics of acyclovir amino acid ester prodrugs in the anterior chamber: evaluation of their utility in treating ocular HSV infections. Int J Pharm. 2008;359:15–24.
Beutner KR, Friedman DJ, Forszpaniak C, Andersen PL, Wood MJ. Valaciclovir compared with acyclovir for improved therapy for herpes zoster in immunocompetent adults. Antimicrob Agents Chemother. 1995;39:1546–53.
Urtti A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv Drug Deliv Rev. 2006;58:1131–5.
Bourlais CL, Acar L, Zia H, Sado PA, Needham T, Leverge R. Ophthalmic drug delivery systems-recent advances. Prog Retin Eye Res. 1998;17:33–58.
Kompella UB, Kadam RS, Lee VH. Recent advances in ophthalmic drug delivery. Ther Deliv. 2010;3:435–56.
Willoughby CE, Ponzin D, Ferrari S, Lobo A, Landau K, Omidi Y. Clin Exp Ophthalmol. 2010;38:2–11.
DelMonte DW, Kim T. Anatomy and physiology of the cornea. J Cataract Refract Surg. 2011;37:588–98.
Toropainen E, Ranta VP, Vellonen KS, et al. Paracellular and passive transcellular permeability in immortalized human corneal epithelial cell culture model. Eur J Pharm Sci. 2003;20:99–106.
Komarova Y, Malik AB. Regulation of endothelial permeability via paracellular and transcellular transport pathways. Annu Rev Physiol. 2010;72:463–93.
Kam KR, Walsh LA, Bock SM, et al. Nanostructure-mediated transport of biologics across epithelial tissue: enhancing permeability via nanotopography. Nano Lett. 2013;13:164–71.
Mehnert W, Mader K. Solid lipid nanoparticles: production, characterization and applications. Adv Drug Deliv Rev. 2001;47:165–96.
Seyfoddin A, Shaw J, Al-Kassas R. Solid lipid nanoparticles for ocular drug delivery. Drug Deliv. 2010;17:467–89.
Puglia C, Offerta A, Carbone C, Bonina F, Pignatello R, Puglisi G. Lipid nanocarriers (LNC) and their applications in ocular drug delivery. Curr Med Chem. 2015;22:1589–602.
Liu D, Chen L, Jiang S, et al. Formulation and characterization of hydrophilic drug diclofenac sodium-loaded solid lipid nanoparticles based on phospholipid complexes technology. J Liposome Res. 2014;24:17–26.
Basaran E, Demirel M, Sirmagul B, Yazan Y. Cyclosporine-A incorporated cationic solid lipid nanoparticles for ocular delivery. J Microencapsul. 2010;27:37–47.
Cavalli R, Gasco MR, Chetoni P, Burgalassi S, Saettone MF. Solid lipid nanoparticles (SLN) as ocular delivery system for tobramycin. Int J Pharm. 2002;238:241–5.
Abul Kalam M, Sultana Y, Ali A, et al. Part II: enhancement of transcorneal delivery of gatifloxacin by solid lipid nanoparticles in comparison to commercial aqueous eye drops. J Biomed Mater Res A. 2013;101:1828–36.
Lutfi G, Muzeyyen D. Preparation and characterization of polymeric and lipid nanoparticles of pilocarpine HCl for ocular application. Pharm Dev Technol. 2013;18:701–9.
Leonardi A, Bucolo C, Drago F, Salomone S, Pignatello R. Cationic solid lipid nanoparticles enhance ocular hypotensive effect of melatonin in rabbit. Int J Pharm. 2015;478:180–6.
Li R, Jiang S, Liu D, et al. A potential new therapeutic system for glaucoma: solid lipid nanoparticles containing methazolamide. J Microencapsul. 2011;28:134–41.
Mohanty B, Majumdar DK, Mishra SK, Panda AK, Patnaik S. Development and characterization of itraconazole-loaded solid lipid nanoparticles for ocular delivery. Pharm Dev Technol. 2015;20:458–64.
Shah KA, Date AA, Joshi MD, Patravale VB. Solid lipid nanoparticles (SLN) of tretinoin: potential in topical delivery. Int J Pharm. 2007;345:163–71.
Trotta M, Debernardi F, Caputo O. Preparation of solid lipid nanoparticles by a solvent emulsification-diffusion technique. Int J Pharm. 2003;257:153–60.
ICCVAM-Recommended test method protocol: Hen’s egg test-chorioallantoic membrane (HET-CAM) test method. https://ntp.niehs.nih.gov/iccvam/docs/protocols/ivocular-hetcam.pdf. Accessed on 26 March 2015.
Monti D, Chetoni P, Burgalass S, Najarro M, Saettone MF. Increased corneal hydration induced by potential ocular penetration enhancers: assessment by differential scanning calorimetry (DSC) and by desiccation. Int J Pharm. 2002;232:139–47.
Wilhelmus KR. The Draize eye test. Surv Ophthalmol. 2001;45:493–515.
Lou J, Hu W, Tian R, et al. Optimization and evaluation of a thermoresponsive ophthalmic in situ gel containing curcumin-loaded albumin nanoparticles. Int J Nanomedicine. 2014;9:2517–25.
Li Q, Li Z, Zeng W, et al. Proniosome-derived niosomes for tacrolimus topical ocular delivery: in vitro cornea permeation, ocular irritation, and in vivo anti-allograft rejection. Eur J Pharm Sci. 2014;62:115–23.
Montenegro L, Bucolo C, Puglisi G. Enhancer effects on in vitro corneal permeation of timolol and acyclovir. Pharmazie. 2003;58:497–501.
Kumar R, Nagarwal RC, Dhanawat M, Pandit JK. In-vitro and in-vivo study of indomethacin loaded gelatin nanoparticles. J Biomed Nanotechnol. 2011;7:325–33.
Lim LT, Ah-kee EY, Collins CE. Common eye drops and their implications for pH measurements in the management of chemical eye injuries. Int J Ophthalmol. 2014;7:1067–8.
Luan X, Skupin M, Siepmann J, Bodmeier R. Key parameters affecting the initial release (burst) and encapsulation efficiency of peptide-containing poly(lactide-co-glycolide) microparticles. Int J Pharm. 2006;324:168–75.
Dash S, Murthy PN, Nath L, Chowdhury P. Kinetic modeling on drug release from controlled drug delivery system. Acta Pol Pharm. 2010;67:217–23.
Kumar R, Sinha VR. Preparation and optimization of voriconazole microemulsion for ocular delivery. Colloids Surf B: Biointerfaces. 2014;117:82–8.
Hamalainen KM, Kontturi K, Auriola S, Murtomaki L, Urtti A. Estimation of pore size and pore density of biomembranes from permeability measurements of polyethylene glycols using an effusion-like approach. J Control Release. 1997;49:97–104.
Saettone MF, Cheton P, Cerbai R, Mazzanti G, Braghiroli L. Evaluation of ocular permeation enhancers: i effects on corneal transport of four β-blockers, and in vitro/in vivo toxic activity. Int J Pharm. 1996;142:103–13.
Acknowledgments
The authors are thankful to University Grant Commission (UGC), New Delhi, India for providing financial support to this research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Kumar, R., Sinha, V.R. Lipid Nanocarrier: an Efficient Approach Towards Ocular Delivery of Hydrophilic Drug (Valacyclovir). AAPS PharmSciTech 18, 884–894 (2017). https://doi.org/10.1208/s12249-016-0575-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-016-0575-2