Abstract
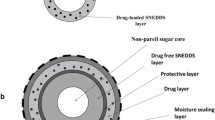
The objective of the present study was to evaluate the feasibility of using model drug metoprolol succinate (MS) as a pore former to modify the initial lag phase (i.e., a slow or non-release phase in the first 1–2 h) associated with the drug release from coated pellets. MS-layered cores with high drug-layering efficiency (97% w/w) were first prepared by spraying a highly concentrated drug aqueous solution (60% w/w, 70°C) on non-pareils without using other binders. The presence of MS in ethylcellulose (EC) coating solution significantly improved the coating process by reducing pellets sticking, which often occurs during organic coating. There may be a maximum physical compatibility of MS with EC, and the physical state of the drug in the functional coating layer of EC/MS (80:20) was simultaneously crystalline and non-crystalline (amorphous or solid molecule solution). The lag phase associated with hydroxypropylcellulose (HPC) as a pore former was not observed when MS was used as a pore former. The drug release from EC/MS-coated pellets was pH independent, inversely proportional to the coating levels, and directly related to the pore former levels. The functional coating layer with MS as a pore former was not completely stabilized without curing. Curing at 60°C for 1 day could substantially improve the stability of EC/MS-coated pellets. The physical state of the drug in the free film of EC/MS (85:15) changed partially from amorphous to crystal when cured at 60°C for 1 day, which should be attributed to the incompatibility of the drug with EC.













Similar content being viewed by others
REFERENCES
Bodmeier R. Tableting of coated pellets. Eur J Pharm Biopharm. 1997;43:1–8.
Appel LE, Beyerinck RA, Chidlaw MB, Curatolo WJ, Friesen DT, Smith KL, Thombre AG. Hydrogel-driven drug dosage form. In: US Patent 2011/0182947 A1; 2011.
Lindstedt B, Ragnarsson G, Hjärtstam J. Osmotic pumping as a release mechanism for membrane-coated drug formulations. Int J Pharm. 1989;56:261–8.
Ragnarsson G, Sandberg A, Johansson MO, Lindstedt B, Sjögren J. In vitro release characteristics of a membrane-coated pellet formulation—influence of drug solubility and particle size. Int J Pharm. 1992;79:223–32.
Marucci M, Ragnarsson G, von Corswant C, Welinder A, Jarke A, Iselau F, et al. Polymer leaching from film coating: effects on the coating transport properties. Int J Pharm. 2011;411:43–8.
Haddish-Berhane N, Jeong SH, Haghighi K, Park K. Modeling film-coat non-uniformity in polymer coated pellets: a stochastic approach. Int J Pharm. 2006;323:64–71.
Ensslin S, Moll KP, Paulus K, Mader K. New insight into modified release pellets-Internal structure and drug release mechanism. J Control Release. 2008;128:149–56.
Kaunisto E, Marucci M, Borgguist P, Axelsson A. Mechanistic modeling of drug release from polymer-coated and swelling and dissolving polymer matrix systems. Int J Pharm. 2011;418:54–77.
Appel LE, Friesen DT, Herbig SM, Thombre AG. Controlled release dosage forms combining immediate release and sustained release of low-solubility drug. In: US Patent 2008/0299188 A1; 2008.
Avramoff A, Domb AJ. In-vitro and in-vivo characteristics of a modified-release double-pulse formulation for a water soluble drug. Int J Clin Pharmacol Ther. 2010;48:250–8.
Verma RK, Garg S. Development and evaluation of osmotically controlled oral drug delivery system of glipizide. Eur J Pharm Biopharm. 2004;57:513–25.
Kumaravelrajan R, Narayanan N, Suba V. Development and evaluation of controlled porosity osmotic pump for nifedipine and metoprolol combination. Lipids Health Dis. 2011;10:51–64.
Gunder W, Lippold BH, Lippold BC. Release of drugs from ethyl cellulose microcapsules (diffusion pellets) with pore formers and pore fusion. Eur J Pharm Sci. 1995;3:203–14.
Siepmann F, Hoffmann A, Leclercq B, Carlin B, Siepmann J. How to adjust desired drug release patterns from ethylcellulose-coated dosage forms. J Control Release. 2007;119:182–9.
Bodmeier R, Paeratakul O. Constant potassium chloride release from microporous membrane-coated tablets prepared with aqueous colloidal polymer dispersions. Pharm Res. 1991;8:355–9.
Lin WJ, Shiue GR. Elucidation of two water leachable polymers impact on microporous membrane performance and drug permeation. J Memb Sci. 2011;373:189–95.
Tuntikulwattana S, Mitrevej A, Kerdcharoen T, Williams DB, Sinchaipanid N. Development and optimization of micro/nanoporous osmotic pump tablets. AAPS PharmSciTech. 2010;11:924–35.
Kim JE, Kim SR, Lee SH, Lee CH, Kim DD. The effect of pore formers on the controlled release of cefadroxil from a polyurethane matrix. Int J Pharm. 2000;201:29–36.
Heckötter UM, Larsson A, Sriamornsak P, Kumpugdee-Vollrath M. Effect of annealing time and addition of lactose on release of a model substance from Eudragit® RS coated pellets produced by a fluidized bed coater. Chem Eng Res Des. 2011;89:697–705.
Mishra M, Mishra B. Design and evaluation of microporous membrane coated matrix tablets for a highly water soluble drug. Chem Pharm Bull (Tokyo). 2010;58:995–1000.
Zhang X, Wang Y, Wang J, Wang Y, Li S. Effect of pore former on the properties of casted film prepared from blends of Eudragit NE 30 D and Eudragit L 30 D-55. Chem Pharm Bull (Tokyo). 2007;55:1261–3.
Garg A, Gupta M, Bhargava HN. Effect of formulation parameters on the release characteristics of propranolol from asymmetric membrane coated tablets. Eur J Pharm Biopharm. 2007;67:725–31.
Wakode R, Bhanushali R, Bajaj A. Development and evaluation of push-pull based osmotic delivery system for pramipexole. PDA J Pharm Sci Technol. 2008;62:22–31.
Verma RK, Kaushal AM, Garg S. Development and evaluation of extended release formulations of isosorbide mononitrate based on osmotic technology. Int J Pharm. 2003;263:9–24.
Marucci M, Ragnarsson G, Nilsson B, Axelsson A. Osmotic pumping release from ethyl–hydroxypropyl–cellulose-coated pellets: a new mechanistic model. J Control Release. 2010;142:53–60.
Andersson H, Hjärtstam J, Stading M, von Corswant C, Larsson A. Effects of molecular weight on permeability and microstructure of mixed ethyl-hydroxypropyl-cellulose films. Eur J Pharm Sci. 2013;48:240–8.
Lecomte F, Siepmann J, Walther M, MacRae RJ, Bodmeier R. pH-sensitive polymer blends used as coating materials to control drug release from spherical beads: elucidation of the underlying mass transport mechanisms. Pharm Res. 2005;22:1129–41.
Siepmann F, Siepmann J, Walther M, MacRae RJ, Bodmeier R. Polymer blends for controlled release coatings. J Control Release. 2008;125:1–15.
Yang MY, Xie S, Li Q, Wang YL, Chang XY, Shan L, et al. Effects of polyvinylpyrrolidone both as a binder and pore-former on the release of sparingly water-soluble topiramate from ethylcellulose coated pellets. Int J Pharm. 2014;465:187–96.
Hema R, Preeti P, Ashwini S, Ulrich PH, Rosario L, Jayanthi IC. Modulated release metoprolol succinate formulation based on ionic interactions: in vivo proof of concept. J Control Release. 2006;111:65–72.
Kendall MJ, Maxwell SR, Sandberg A, Westergren G. Controlled release metoprolol, clinical pharmacokinetic and therapeutic implications. Clin Pharamcokinet. 1991;5:319–30.
Sinchaipanid N, Chitropas P, Mitrevej A. Influences of layering process on theophylline pellet characteristics. Pharm Dev Technol. 2004;9:163–70.
Baki G, Bajdik J, Djuric D, Knop K, Kleinebudde P, Pintye-Hódi K. Role of surface free energy and spreading coefficient in the formulation of active agent-layered pellets. Eur J Pharm Biopharm. 2010;74:324–31.
Ahmad H, Khalifeh I, Alkhalidi B, Aiedeh K, AlKhatib HS. Application of active layering and coating techniques in the development of a multiparticulate, controlled release dosage form of a high-dose, highly soluble drug. Pharm Dev Technol. 2014;19:556–64.
Lee MJ, Seo DY, Lee HE, Wang IC, Kim WS, Jeong MY, et al. In line NIR quantification of film thickness on pharmaceutical pellets during a fluid bed coating process. Int J Pharm. 2011;403:66–72.
Ho L, Cuppok Y, Muschert S, Gordon KC, Pepper M, Shen YC, et al. Effects of film coating thickness and drug layer uniformity on vitro drug release from sustained-release coated pellets: a case study using terahertz pulsed imaging. Int J Pharm. 2009;382:151–9.
Erdmann H, Gebert S, Kolter K, Schepky G. Studies on modifying the tackiness and drug release of Kollicoat EMM 30 D coatings. Drug Dev Ind Pharm. 2003;29:429–40.
Nimkulrat S, Suchiva K, Phinyocheep P, Puttipipatkhachorn S. Influence of selected surfactants on the tackiness of acrylic polymer films. Int J Pharm. 2004;287:27–37.
Wan LSC, Lai WF. The influence of antitack additives on drug release from film-coated granules. Int J Pharm. 1993;94:39–47.
Felton LA, McGinity JW. Influence of insoluble excipients on film coating systems. Drug Dev Ind Pharm. 2002;28:225–43.
Chan LW, Wong TW, Chua PC, York P, Heng PWS. Anti-tack action of polyvinylpyrrolidone on hydroxypropylmethylcellulose solution. Chem Pharm Bull. 2003;51:107–12.
Marucci M, Arnehed J, Jarke A, Matic H, Nicholas M, Boissier C, et al. Effect of the manufacturing conditions on the structure and permeability of polymer films intended for coating undergoing phase separation. Eur J Pharm Biopharm. 2013;83:301–6.
Marucci M, Hjartstam J, Ragnarsson G, Iselau F, Axelsson A. Coated formulation: new insight into the release mechanism and changes in the film properties with a novel release cell. J Control Release. 2009;139:206–12.
Dashevskya A, Kolterb K, Bodmeier R. pH-independent release of a basic drug from pellets coated with the extended release polymer dispersion Kollicoat® SR 30 D and the enteric polymer dispersion Kollicoat® MAE 30 DP. Eur J Pharm Biopharm. 2004;58:45–9.
Wesseling M, Bodmeier R. Drug release from beads coated with an aqueous colloidal ethylcellulose dispersion, Aquacoat®, or an organic ethylcellulose solution. Eur J Pharm Biopharm. 1999;47:33–8.
ACKNOWLEDGMENTS
The authors are grateful to the Important National Science & Technology Specific Projects (Grant no. 2012ZX09301003-001-009) and the State Key Laboratory of Antitoxic Drugs and Toxicology for their financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Y., Dai, J., Chang, X. et al. Model Drug as Pore Former for Controlled Release of Water-Soluble Metoprolol Succinate from Ethylcellulose-Coated Pellets Without Lag Phase: Opportunities and Challenges. AAPS PharmSciTech 16, 35–44 (2015). https://doi.org/10.1208/s12249-014-0197-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-014-0197-5