Abstract
CYP3A is one of the most important classes of enzymes and is involved in the metabolism of over 70% drugs. While several selective CYP3A4 inhibitors have been identified, the search for a selective CYP3A5 inhibitor has turned out to be rather challenging. Recently, several selective CYP3A5 inhibitors have been identified through high-throughput screening of ~ 11,000 compounds and hit expansion using human recombinant enzymes. We set forth to characterize the three most selective CYP3A5 inhibitors in a more physiologically relevant system of human liver microsomes to understand if these inhibitors can be used for reaction phenotyping studies in drug discovery settings. Gomisin A and T-5 were used as selective substrate reactions for CYP3A4 and CYP3A5 to determine IC50 values of the two enzymes. The results showed that clobetasol propionate and loteprednol etabonate were potent and selective CYP3A5 reversible inhibitors with selectivity of 24-fold against CYP3A4 and 39-fold or more against the other major CYPs. The selectivity of difluprednate in HLM is much weaker than that in the recombinant enzymes due to hydrolysis of the acetate group in HLM. Based on the selectivity data, loteprednol etabonate can be utilized as an orthogonal approach, when experimental fraction metabolized of CYP3A5 is greater than 0.5, to understand CYP3A5 contribution to drug metabolism and its clinical significance. Future endeavors to identify even more selective CYP3A5 inhibitors are warranted to enable accurate determination of CYP3A5 contribution to metabolism versus CYP3A4.
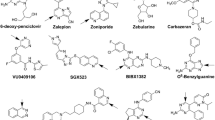
Graphical Abstract








Similar content being viewed by others
Abbreviations
- ACN:
-
Acetonitrile
- CLint :
-
Intrinsic clearance
- CYP:
-
Cytochrome P450
- DDI:
-
Drug-drug interactions
- FDA:
-
U.S. Food and Drug Administration
- f m :
-
Fraction metabolized
- HLM:
-
Human liver microsomes
- IC50 :
-
Half-maximal inhibitory concentration
- IS:
-
Internal standards
- K m :
-
The concentration of substrate which permits the enzyme to achieve half Vmax
- k obs :
-
Inactivation rate constant
- LC–MS/MS:
-
Liquid chromatography with tandem mass spectrometry
- MgCl2 :
-
Magnesium chloride
- MRM:
-
Multiple-reaction monitoring
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate, reduced form
- QSAR:
-
Quantitative structure–activity relationship
- rCYP:
-
Recombinant cytochrome P450
- RT:
-
Room temperature
- TDI:
-
Time-dependent inhibition
- V max :
-
The maximum rate of reaction
References
Saravanakumar A, Sadighi A, Ryu R, Akhlaghi F. Physicochemical properties, biotransformation, and transport pathways of established and newly approved medications: a systematic review of the top 200 most prescribed drugs vs. the FDA-approved drugs between 2005 and 2016. Clin Pharmacokinet. 2019;58(10):1281–94. https://doi.org/10.1007/s40262-019-00750-8.
Daly AK. Significance of the minor cytochrome P450 3A isoforms. Clin Pharmacokinet. 2006;45(1):13–31. https://doi.org/10.2165/00003088-200645010-00002.
Hines RN. Ontogeny of human hepatic cytochromes P450. J Biochem Mol Toxicol. 2007;21(4):169–75. https://doi.org/10.1002/jbt.20179.
Lacroix D, Sonnier M, Moncion A, Cheron G, Cresteil T. Expression of CYP3A in the human liver–evidence that the shift between CYP3A7 and CYP3A4 occurs immediately after birth. Eur J Biochem. 1997;247(2):625–34. https://doi.org/10.1111/j.1432-1033.1997.00625.x.
Wilkening S, Bader A. Differential regulation of CYP3A4 and CYP3A7 by dimethylsulfoxide in primary human hepatocytes. Basic Clin Pharmacol Toxicol. 2004;95(2):92–3. https://doi.org/10.1111/j.1742-7843.2004.950209.x.
Betts S, Björkhem-Bergman L, Rane A, Ekström L. Expression of CYP3A4 and CYP3A7 in human foetal tissues and its correlation with nuclear receptors. Basic Clin Pharmacol Toxicol. 2015;117(4):261–6. https://doi.org/10.1111/bcpt.12392.
Zientek MA, Youdim K. Reaction phenotyping: advances in the experimental strategies used to characterize the contribution of drug-metabolizing enzymes. Drug Metab Dispos. 2015;43(1):163–81. https://doi.org/10.1124/dmd.114.058750.
Leeder JS, Gaedigk R, Marcucci KA, Gaedigk A, Vyhlidal CA, Schindel BP, et al. Variability of CYP3A7 expression in human fetal liver. J Pharmacol Exp Ther. 2005;314:626–35. https://doi.org/10.1124/jpet.105.086504.
Burk O, Tegude H, Koch I, Hustert E, Wolbold R, Glaeser H, et al. Molecular mechanisms of polymorphic CYP3A7 expression in adult human liver and intestine. J Biol Chem. 2002;277:24280–8. https://doi.org/10.1074/jbc.M202345200.
Langman L, van Gelder T, van Schaik RHN. Chapter 5 - Pharmacogenomics aspect of immunosuppressant therapy. In: Oellerich M, Dasgupta A, editors. Personalized immunosuppression in transplantation. San Diego: Elsevier; 2016. p. 109–24.
Hsu M-H, Johnson EF. Active-site differences between substrate-free and ritonavir-bound cytochrome P450 (CYP) 3A5 reveal plasticity differences between CYP3A5 and CYP3A4. J Biol Chem. 2019;294(20):8015–22. https://doi.org/10.1074/jbc.RA119.007928.
Yano JK, Wester MR, Schoch GA, Griffin KJ, Stout CD, Johnson EF. The structure of human microsomal cytochrome P450 3A4 determined by X-ray crystallography to 2.05-Å resolution*. J Biol Chem. 2004;279(37):38091–4. https://doi.org/10.1074/jbc.C400293200.
Tseng E, Walsky RL, Luzietti RA Jr, Harris JJ, Kosa RE, Goosen TC, et al. Relative contributions of cytochrome CYP3A4 versus CYP3A5 for CYP3A-cleared drugs assessed in vitro using a CYP3A4-selective inactivator (CYP3cide). Drug Metab Dispos. 2014;42:1163–73. https://doi.org/10.1124/dmd.114.057000.
Vourvahis M, McFadyen L, Heera J, Clark A. Clinical relevance of CYP3A5 genotype on maraviroc exposures. Drug Metab Dispos. 2015;43(5):771–2. https://doi.org/10.1124/dmd.115.063321.
Dennison JB, Kulanthaivel P, Barbuch RJ, Renbarger JL, Ehlhardt WJ, Hall SD. Selective metabolism of vincristine in vitro by CYP3A5. Drug Metab Dispos. 2006;34(8):1317–27. https://doi.org/10.1124/dmd.106.009902.
Khan AR, Raza A, Firasat S, Abid A. CYP3A5 gene polymorphisms and their impact on dosage and trough concentration of tacrolimus among kidney transplant patients: a systematic review and meta-analysis. Pharmacogenomics J. 2020;20(4):553–62. https://doi.org/10.1038/s41397-019-0144-7.
Jin Y, Wang YH, Miao J, Li L, Kovacs RJ, Marunde R, et al. Cytochrome P450 3A5 genotype is associated with verapamil response in healthy subjects. Clin Pharmacol Ther. 2007;82:579–85. https://doi.org/10.1038/sj.clpt.6100208.
Zhu HJ, Yuan SH, Fang Y, Sun XZ, Kong H, Ge WH. The effect of CYP3A5 polymorphism on dose-adjusted cyclosporine concentration in renal transplant recipients: a meta-analysis. Pharmacogenomics J. 2011;11(3):237–46. https://doi.org/10.1038/tpj.2010.26.
Chen L, Prasad GVR. CYP3A5 polymorphisms in renal transplant recipients: influence on tacrolimus treatment. Pharmacogenomics Pers Med. 2018;11:23–33. https://doi.org/10.2147/pgpm.s107710.
Skiles JL, Chiang C, Li CH, Martin S, Smith EL, Olbara G, et al. CYP3A5 genotype and its impact on vincristine pharmacokinetics and development of neuropathy in Kenyan children with cancer. Pediatr Blood Cancer. 2018;65(3):1–14. https://doi.org/10.1002/pbc.26854.
Noll EM, Eisen C, Stenzinger A, Espinet E, Muckenhuber A, Klein C, et al. CYP3A5 mediates basal and acquired therapy resistance in different subtypes of pancreatic ductal adenocarcinoma. Nat Med. 2016;22:278–87. https://doi.org/10.1038/nm.4038.
Mao Q, Wang L, Liang Y, Dong G, Xia W, Hu J, et al. CYP3A5 suppresses metastasis of lung adenocarcinoma through ATOH8/Smad1 axis. Am J Cancer Res. 2020;10:3194–211.
Gorjala P, Kittles RA, Goodman OB Jr, Mitra R. Role of CYP3A5 in modulating androgen receptor signaling and its relevance to African American men with prostate cancer. Cancers. 2020;12(4):989–1005. https://doi.org/10.3390/cancers12040989.
Jiang F, Chen L, Yang Y-C, Wang X-m, Wang R-Y, Li L, et al. CYP3A5 functions as a tumor suppressor in hepatocellular carcinoma by regulating mTORC2/Akt signaling. Cancer Res. 2015;75:1470–81. https://doi.org/10.1158/0008-5472.can-14-1589.
Buck E, Sprick M, Gaida MM, Grüllich C, Weber TF, Herpel E, et al. Tumor response to irinotecan is associated with CYP3A5 expression in colorectal cancer. Oncol Lett. 2019;17:3890–8. https://doi.org/10.3892/ol.2019.10043.
Werk AN, Cascorbi I. Functional gene variants of CYP3A4. Clin Pharmacol Ther. 2014;96(3):340–8. https://doi.org/10.1038/clpt.2014.129.
Wang J, Buchman CD, Seetharaman J, Miller DJ, Huber AD, Wu J, et al. Unraveling the structural basis of selective inhibition of human cytochrome P450 3A5. J Am Chem Soc. 2021;143:18467–80. https://doi.org/10.1021/jacs.1c07066.
Li X, Song X, Kamenecka TM, Cameron MD. Discovery of a highly selective CYP3A4 inhibitor suitable for reaction phenotyping studies and differentiation of CYP3A4 and CYP3A5. Drug Metab Dispos. 2012;40(9):1803–9. https://doi.org/10.1124/dmd.112.046144.
Walsky RL, Obach RS, Hyland R, Kang P, Zhou S, West M, et al. Selective mechanism-based inactivation of CYP3A4 by CYP3cide (PF-04981517) and its utility as an in vitro tool for delineating the relative roles of CYP3A4 versus CYP3A5 in the metabolism of drugs. Drug Metab Dispos. 2012;40:1686–97. https://doi.org/10.1124/dmd.112.045302.
Wright WC, Chenge J, Wang J, Girvan HM, Yang L, Chai SC, et al. Clobetasol propionate is a heme-mediated selective inhibitor of human cytochrome P450 3A5. J Med Chem. 2020;63:1415–33. https://doi.org/10.1021/acs.jmedchem.9b02067.
Di L. Reaction phenotyping to assess victim drug-drug interaction risks. Expert Opin Drug Discovery. 2017;12(11):1105–15. https://doi.org/10.1080/17460441.2017.1367280.
Wu J, Guan X, Dai Z, He R, Ding X, Yang L, et al. Molecular probes for human cytochrome P450 enzymes: recent progress and future perspectives. Coord Chem Rev. 2021;427:213600. https://doi.org/10.1016/j.ccr.2020.213600.
Li X, Jeso V, Heyward S, Walker GS, Sharma R, Micalizio GC, et al. Characterization of T-5 N-oxide formation as the first highly selective measure of CYP3A5 activity. Drug Metab Dispos. 2014;42:334–42. https://doi.org/10.1124/dmd.113.054726.
Wu J-J, Cao Y-F, Feng L, He Y-Q, Hong JY, Dou T-Y, et al. A Naturally occurring isoform-specific probe for highly selective and sensitive detection of human cytochrome P450 3A5. J Med Chem. 2017;60:3804–13. https://doi.org/10.1021/acs.jmedchem.7b00001.
Wu JJ, Ge GB, He YQ, Wang P, Dai ZR, Ning J, et al. Gomisin A is a novel isoform-specific probe for the selective sensing of human cytochrome P450 3A4 in liver microsomes and living cells. AAPS J. 2016;18:134–45. https://doi.org/10.1208/s12248-015-9827-4.
Cao YF, Zhang YY, Li J, Ge GB, Hu D, Liu HX, et al. CYP3A catalyses schizandrin biotransformation in human, minipig and rat liver microsomes. Xenobiotica. 2010;40:38–47. https://doi.org/10.3109/00498250903366052.
Ge G-B, Ning J, Hu L-H, Dai Z-R, Hou J, Cao Y-F, et al. A highly selective probe for human cytochrome P450 3A4: isoform selectivity, kinetic characterization and its applications. Chem Commun. 2013;49:9779–81. https://doi.org/10.1039/C3CC45250F.
Ning J, Yu ZL, Hu LH, Wang C, Huo XK, Deng S, et al. Characterization of phase I metabolism of resibufogenin and evaluation of the metabolic effects on its antitumor activity and toxicity. Drug Metab Dispos. 2015;43:299–308. https://doi.org/10.1124/dmd.114.060996.
Zientek M, Youdim K. Simultaneous determination of multiple CYP inhibition constants using a cocktail-probe approach. Methods Mol Biol (Clifton, NJ). 2013;987:11–23. https://doi.org/10.1007/978-1-62703-321-3_2.
Zientek M, Miller H, Smith D, Dunklee MB, Heinle L, Thurston A, et al. Development of an in vitro drug-drug interaction assay to simultaneously monitor five cytochrome P450 isoforms and performance assessment using drug library compounds. J Pharmacol Toxicol Methods. 2008;58:206–14. https://doi.org/10.1016/j.vascn.2008.05.131.
Yates P, Eng H, Di L, Obach RS. Statistical methods for analysis of time-dependent inhibition of cytochrome p450 enzymes. Drug Metab Dispos. 2012;40(12):2289–96. https://doi.org/10.1124/dmd.112.047233.
Lu Y, Hendrix CW, Bumpus NN. Cytochrome P450 3A5 plays a prominent role in the oxidative metabolism of the anti-human immunodeficiency virus drug maraviroc. Drug Metab Dispos. 2012;40(12):2221–30. https://doi.org/10.1124/dmd.112.048298.
Author information
Authors and Affiliations
Contributions
Substantial contributions to the conception or design of the work or the acquisition, analysis, or interpretation of data for the work: JC, LT, SJ, MH, GG, ED, DM, GB, YC, and LD.
Drafting the work or revising it critically for important intellectual content: JC, LT, SJ, ED, and LD.
Final approval of the version to be published: JC, LT, SJ, MH, GG, ED, DM, GB, YC, and LD.
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: JC, LT, SJ, MH, GG, ED, DM, GB, YC, and LD.
Corresponding author
Ethics declarations
Conflict of Interest
The authors are employees of Pfizer Inc., New York, NY, USA, and may hold Pfizer stocks.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, J., Tang, L.W.T., Jordan, S. et al. Characterization of CYP3A5 Selective Inhibitors for Reaction Phenotyping of Drug Candidates. AAPS J 26, 26 (2024). https://doi.org/10.1208/s12248-024-00894-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-024-00894-x