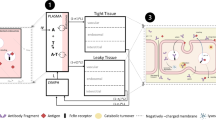
Abstract
An integrated physiologically based modeling framework is presented for predicting pharmacokinetics and bioavailability of subcutaneously administered monoclonal antibodies in cynomolgus monkeys, based on in silico structure-derived metrics characterizing antibody size, overall charge, local charge, and hydrophobicity. The model accounts for antibody-specific differences in pinocytosis, transcapillary transport, local lymphatic uptake, and pre-systemic degradation at the subcutaneous injection site and reliably predicts the pharmacokinetics of five different wild-type mAbs and their Fc variants following intravenous and subcutaneous administration. Significant associations were found between subcutaneous injection site degradation rate and the antibody’s local positive charge of its complementarity-determining region (R = 0.56, p = 0.0012), antibody pinocytosis rate and its overall positive charge (R = 0.59, p = 0.00063), and antibody paracellular transport and its overall charge together with hydrophobicity (R = 0.63, p = 0.00096). Based on these results, population simulations were performed to predict the relationship between bioavailability and antibody local positive charge. In addition, model simulations were conducted to calculate the relative contribution of absorption pathways (lymphatic and blood), pre-systemic degradation pathways (interstitial and lysosomal), and the influence of injection site lymph flow on antibody bioavailability and pharmacokinetics. The proposed physiologically based modeling framework integrates fundamental mechanisms governing antibody subcutaneous absorption and disposition, with structured-based physiochemical properties, to predict antibody bioavailability and pharmacokinetics in vivo.
Graphical Abstract





Similar content being viewed by others
References
Collins DS, Sánchez-Félix M, Badkar AV, Mrsny R. Accelerating the development of novel technologies and tools for the subcutaneous delivery of biotherapeutics. J Control Release. 2020;321(January):475–82.
Sánchez-Félix M, Burke M, Chen HH, Patterson C, Mittal S. Predicting bioavailability of monoclonal antibodies after subcutaneous administration: open innovation challenge. Adv Drug Deliv Rev. 2020;167:66–77.
Richter WF, Bhansali SG, Morris ME. Mechanistic determinants of biotherapeutics absorption following SC administration. AAPS J. 2012;14(3):559–70.
Datta-Mannan A, Estwick S, Zhou C, Choi H, Douglass NE, Witcher DR, et al. Influence of physiochemical properties on the subcutaneous absorption and bioavailability of monoclonal antibodies. MAbs. 2020;12(1):1–14.
Zheng Y, Tesar DB, Benincosa L, Birnböck H, Boswell CA, Bumbaca D, et al. Minipig as a potential translatable model for monoclonal antibody pharmacokinetics after intravenous and subcutaneous administration. MAbs. 2012;4(2):243–55.
MacH H, Gregory SM, MacKiewicz A, Mittal S, Lalloo A, Kirchmeier M, et al. Electrostatic interactions of monoclonal antibodies with subcutaneous tissue. Ther Deliv. 2011;2(6):727–36.
Deng R, Meng YG, Hoyte K, Lutman J, Lu Y, Iyer S, et al. Subcutaneous bioavailability of therapeutic antibodies as a function of FcRn binding affinity in mice. MAbs. 2012;4(1):101–9.
Richter WF, Christianson GJ, Frances N, Grimm HP, Proetzel G, Roopenian DC. Hematopoietic cells as site of first-pass catabolism after subcutaneous dosing and contributors to systemic clearance of a monoclonal antibody in mice. MAbs. 2018;10(5):803–13.
Datta-Mannan A, Witcher DR, Lu J, Wroblewski VJ. Influence of improved FcRn binding on the subcutaneous bioavailability of monoclonal antibodies in cynomolgus monkeys. MAbs. 2012;4(2):267–73.
Nnane IP, Han C, Jiao Q, Tam SH, Davis HM, Xu Z. Modification of the Fc region of a human anti-oncostatin M monoclonal antibody for higher affinity to FcRn receptor and extension of half-life in cynomolgus monkeys. Basic Clin Pharmacol Toxicol. 2017;121:13–21.
Richter WF, Grimm HP, Gouy MH, Søgaard S, Kreuzer C, Wessels U, et al. Subcutaneous site-of-absorption study with the monoclonal antibody tocilizumab in minipigs: administration behind ear translates best to humans. AAPS J. 2020;22(3):1–10.
Bender C, Eichling S, Franzen L, Herzog V, Ickenstein LM, Jere D, et al. Evaluation of in vitro tools to predict the in vivo absorption of biopharmaceuticals following subcutaneous administration. J Pharmaceut Sci. 2022
Baxter LT, Zhu H, Jain RK, Zhu H, Mackensen DG, Butler WF. Biodistribution of monoclonal antibodies: scale-up from mouse to human using a physiologically based pharmacokinetic model. Cancer Res. 1995;55(20):4611–22.
Garg A, Balthasar JP. Physiologically-based pharmacokinetic (PBPK) model to predict IgG tissue kinetics in wild-type and FcRn-knockout mice. J Pharmacokinet Pharmacodyn. 2007;34:687–709.
Shah DK, Betts AM. Towards a platform PBPK model to characterize the plasma and tissue disposition of monoclonal antibodies in preclinical species and human. J Pharmacokinet Pharmacodyn. 2012;39(1):67–86.
Glassman PM, Balthasar JP. Physiologically-based pharmacokinetic modeling to predict the clinical pharmacokinetics of monoclonal antibodies. J Pharmacokinet Pharmacodyn. 2016;43(4):427–46.
Hu S, Datta-Mannan A, D’Argenio DZ. Physiologically based modeling to predict monoclonal antibody pharmacokinetics in humans from in vitro physiochemical properties. In MAbs: Taylor & Francis 2022;14(1):2056944.
Offman E, Edginton AN. A PBPK workflow for first-in-human dose selection of a subcutaneously administered pegylated peptide. J Pharmacokinet Pharmacodyn. 2015;42(2):135–50.
Gill KL, Gardner I, Li L, Jamei M. A Bottom-up whole-body physiologically based pharmacokinetic model to mechanistically predict tissue distribution and the rate of subcutaneous absorption of therapeutic proteins. AAPS J. 2016;18(1):156–70.
Li Z, Yu X, Li Y, Verma A, Chang HP, Shah DK. A two-pore physiologically based pharmacokinetic model to predict subcutaneously administered different-size antibody/antibody fragments. AAPS J. 2021;23(3):1–13
Datta-Mannan A, Chow CK, Dickinson C, Driver D, Lu J, Witcher DR, et al. FcRn affinity-pharmacokinetic relationship of five human IgG4 antibodies engineered for improved in vitro FcRn binding properties in cynomolgus monkeys. Drug Metab Dispos. 2012;40(8):1545–55.
Raybould MIJ, Marks C, Krawczyk K, Taddese B, Nowak J, Lewis AP, et al. Five computational developability guidelines for therapeutic antibody profiling. Proc Natl Acad Sci. 2019;116(10):4025–30.
Molecular Operating Environment (MOE), 2022.02 Chemical Computing Group ULC, 1010 Sherbooke St. West, Suite #910, Montreal, QC, Canada, H3A 2R7, 2022.
Hu S, D’Argenio DZ. Predicting monoclonal antibody pharmacokinetics following subcutaneous administration via whole-body physiologically-based modeling. J Pharmacokinet Pharmacodyn. 2020;47(5):385–409.
Jones HM, Zhang Z, Jasper P, Luo H, Avery LB, King LE, et al. A Physiologically-based pharmacokinetic model for the prediction of monoclonal antibody pharmacokinetics from in vitro data. CPT Pharmacometrics Syst Pharmacol. 2019;8:738–47.
Mandikian D, Figueroa I, Oldendorp A, Rafidi H, Ulufatu S, Schweiger MG, et al. Tissue physiology of cynomolgus monkeys: cross-species comparison and implications for translational pharmacology. AAPS J. 2018;20(6):1–13.
Glassman PM, Chen Y, Balthasar JP. Scale-up of a physiologically-based pharmacokinetic model to predict the disposition of monoclonal antibodies in monkeys. J Pharmacokinet Pharmacodyn. 2015;42(5):527–40.
Koo B-S, Lee D-H, Kang P, Jeong K-J, Lee S, Kim K, et al. Reference values of hematological and biochemical parameters in young-adult cynomolgus monkey (Macaca fascicularis) and rhesus monkey (Macaca mulatta) anesthetized with ketamine hydrochloride. Lab Anim Res. 2019;35(1):1–6.
Nakayama S, Koie H, Pai C, Ito-Fujishiro Y, Kanayama K, Sankai T, et al. Echocardiographic evaluation of cardiac function in cynomolgus monkeys over a wide age range. Exp Anim. 2020;69(3):336–44.
Gelman S, Fowler KC, Bishop SP, Smith LR. Cardiac output distribution and regional blood flow during hypocarbia in monkeys. J Appl Physiol. 1985;58(4):1225–30.
Datta-Mannan A, Witcher DR, Tang Y, Watkins J, Wroblewski VJ. Monoclonal antibody clearance: impact of modulating the interaction of IgG with the neonatal Fc receptor. J Biol Chem. 2007;282(3):1709–17.
Li T, Balthasar JP. Application of physiologically based pharmacokinetic modeling to predict the effects of FcRn inhibitors in mice, rats, and monkeys. J Pharm Sci. 2019;108(1):701–13.
Hardiansyah D, Ng CM. Minimal physiologically-based pharmacokinetic model to investigate the effect of pH dependent FcRn affinity and the endothelial endocytosis on the pharmacokinetics of anti-VEGF humanized IgG1 antibody in cynomolgus monkey. Eur J Pharm Sci. 2018;125(May):130–41.
Sepp A, Berges A, Sanderson A, Meno-Tetang G. Development of a physiologically based pharmacokinetic model for a domain antibody in mice using the two-pore theory. J Pharmacokinet Pharmacodyn. 2015;42(2):97–109.
Li Z, Shah DK. Two-pore physiologically based pharmacokinetic model with de novo derived parameters for predicting plasma PK of different size protein therapeutics. J Pharmacokinet Pharmacodyn. 2019;46(3):305–18.
Yeung YA, Leabman MK, Marvin JS, Qiu J, Adams CW, Lien S, et al. Engineering human IgG1 affinity to human neonatal Fc receptor: impact of affinity improvement on pharmacokinetics in primates. J Immunol. 2009;182(12):7663–71.
D’Argenio DZ, Alan S, Wang X. ADAPT 5 User’s guide: Pharmacokinetic/Pharmacodynamic Systems Analysis Software. Biomedical Simulations Resources, editor. Los Angeles: Biomedical Simulations Resources; 2009.
Smith B, Kiessling A, Lledo-Garcia R, Dixon KL, Christodoulou L, Catley MC, et al. Generation and characterization of a high affinity anti-human FcRn antibody, rozanolixizumab, and the effects of different molecular formats on the reduction of plasma IgG concentration. MAbs. 2018;10(7):1111–30.
Mezo AR, McDonnell KA, Tan Hehir CA, Low SC, Palombella VJ, Stattel JM, et al. Reduction of IgG in nonhuman primates by a peptide antagonist of the neonatal Fc receptor FcRn. Proc Natl Acad Sci. 2008;105(7):2337–42.
R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2021.
McLennan DN, Porter CJH, Charman SA. Subcutaneous drug delivery and the role of the lymphatics. Drug Discov Today Technol. 2005;2(1):89–96.
Richter WF, Jacobsen B. Subcutaneous absorption of biotherapeutics: knowns and unknowns. Drug Metab Dispos [Internet]. 2014 [cited 2019 Mar 6];42:1881–9. Available from: https://doi.org/10.1124/dmd.114.059238
Zou P, Wang F, Wang J, Lu Y, Tran D, Seo SK. Impact of injection sites on clinical pharmacokinetics of subcutaneously administered peptides and proteins. J Control Release [Internet]. 2021;336(June):310–21. https://doi.org/10.1016/j.jconrel.2021.06.038
Viola M, Sequeira J, Seiça R, Veiga F, Serra J, Santos AC, et al. Subcutaneous delivery of monoclonal antibodies: how do we get there? J Control Release. 2018;286:301–14.
Bumbaca Yadav D, Sharma VK, Andrew Boswell C, Hotzel I, Tesar D, Shang Y, et al. Evaluating the use of antibody variable region (Fv) charge as a risk assessment tool for predicting typical cynomolgus monkey pharmacokinetics. J Biol Chem. 2015;290(50):29732–41.
Datta-Mannan A, Thangaraju A, Leung D, Tang Y, Witcher DR, Lu J, et al. Balancing charge in the complementarity-determining regions of humanized mAbs without affecting pl reduces non-specific binding and improves the pharmacokinetics. MAbs. 2015;7(3):483–93.
Liu S, Verma A, Kettenberger H, Richter WF, Shah DK. Effect of variable domain charge on in vitro and in vivo disposition of monoclonal antibodies. In MAbs: Taylor & Francis 2021;13(1):1993769.
Grinshpun B, Thorsteinson N, Pereira JNS, Rippmann F, Nannemann D, Sood VD, et al. Identifying biophysical assays and in silico properties that enrich for slow clearance in clinical-stage therapeutic antibodies. MAbs. 2021;13(1):1–12.
Sharma VK, Patapoff TW, Kabakoff B, Pai S, Hilario E, Zhang B, et al. In silico selection of therapeutic antibodies for development: Viscosity, clearance, and chemical stability. Proc Natl Acad Sci. 2014;111(52):18601–6.
Ryman JT, Meibohm B. Pharmacokinetics of monoclonal antibodies. CPT Pharmacometrics Syst Pharmacol. 2017;6(9):576–88.
Funding
This work was supported by grants from the National Institutes of Health/ National Institute of Biomedical Imaging and Bioengineering (NIH/ NIBIB) P41-EB001978 and the Alfred E. Mann Institute at USC (DZD).
Author information
Authors and Affiliations
Contributions
S.H., A. D-M, and D.Z.D. designed the research; S.H., A. D-M, and D.Z.D. performed the research; S.H. and D.Z.D. wrote the manuscript; S.H., A. D-M, and D.Z.D. reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hu, S., Datta-Mannan, A. & D’Argenio, D.Z. Monoclonal Antibody Pharmacokinetics in Cynomolgus Monkeys Following Subcutaneous Administration: Physiologically Based Model Predictions from Physiochemical Properties. AAPS J 25, 5 (2023). https://doi.org/10.1208/s12248-022-00772-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-022-00772-4