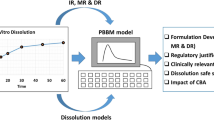
Abstract
Dissolution specifications are often essential in assuring the quality and consistency of therapeutic benefits of drug lots released to the market as in vitro dissolution is often considered to be a surrogate for bioavailability. Despite the importance of demonstrating the clinical relevance of the dissolution specifications, it is often challenging to achieve this goal. In this case study, a modeling and simulation approach was utilized to support the clinical relevance of the dissolution specifications for upadacitinib extended-release tablets. A level A in vitro in vivo correlation was developed and utilized in predicting upadacitinib plasma exposures for formulations which correspond to the upper and lower dissolution limits. Exposure-response models for upadacitinib efficacy and safety in patients with moderate to severe rheumatoid arthritis (RA) were utilized to conduct clinical trial simulations to evaluate the efficacy and safety of formulations at the upper and lower dissolution boundaries. Each simulated clinical trial consisted of three treatment arms: (1) upadacitinib 15 mg QD using the target formulation, (2) upadacitinib 15 mg QD using a formulation at the lower dissolution boundary, and (3) upadacitinib 15 mg QD using a formulation at the upper dissolution boundary. Each simulated trial included 300 patients per arm and simulations were replicated 200 times. Results demonstrated that formulations at the lower and upper dissolution boundaries are predicted to have noninferior efficacy and comparable safety to the target 15 mg extended-release formulation. This approach was successfully utilized in demonstrating the clinical relevance of upadacitinib extended-release tablet dissolution specifications.

Graphical Abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Dissolution specifications are often essential in assuring the quality of the drug lots that are released to the market as dissolution testing is often utilized as a surrogate for in vivo performance and bioavailability of drug products (1,2,3). The dissolution specifications for a drug product are selected to ensure that the released batches of an oral formulation for a drug have acceptable release characteristics leading to the desired pharmacokinetic performance and a consistent clinical profile. Traditionally, the acceptable dissolution limits have been selected based on manufacturing quality considerations to control batch to batch variability, which may or may not be linked to clinical relevance (4). Recently, there has been an increasing interest in utilizing clinically relevant dissolution specifications for drug products (1, 4). Several approaches have been proposed for linking the proposed dissolution specifications to in vivo performance, including in vivo evaluation of variant formulations corresponding to the proposed specifications, use of safe space approach, and developing in vitro in vivo correlation (IVIVC) (4).
With clinically relevant specifications, the dissolution specifications are set based on a clinical outcome (e.g., the bioequivalence criteria or efficacy/safety of formulations at the boundaries) to assure consistent safety and efficacy profiles from lot to lot and within the same lot. Such specifications ensure a more consistent therapeutic effect leading to optimal benefit to the patient. Additionally, clinically relevant specifications might lead to wider dissolution specifications than the traditional approach, which will reduce the probability of rejecting batches that are deemed acceptable from a safety and efficacy point of view (5). Wider dissolution specifications can provide more flexibility in manufacturing as they allow a broader operating range and will reduce the overall cost of the drug on the market by decreasing the number of rejected batches. The benefits of establishing clinically relevant dissolution specifications usually outweigh the cost of the additional studies and efforts that are entailed in setting them (1,2,3).
Upadacitinib is a selective oral Janus kinase (JAK) 1 inhibitor that was approved for the treatment of moderate to severe rheumatoid arthritis (RA) (6, 7). The recommended upadacitinib regimen in RA is 15 mg once daily (QD) administered using an extended-release (ER) formulation (6). Upadacitinib is highly permeable and highly soluble at clinically relevant doses across the pH range of 1–7.5 (8). The pharmacokinetics of upadacitinib have been characterized following the administration of immediate-release capsule and the ER tablet formulations (9,10,11,12,13). Upadacitinib has dose-proportional pharmacokinetics with a terminal half-life of 9–14 h and demonstrated no relevant accumulation in plasma with multiple twice-daily administration of the immediate-release or QD administration of the ER formulations. Upadacitinib is a substrate for metabolism by cytochrome P450 (CYP) 3A and is a substrate for P-glycoprotein (P-gp) and Breast Cancer Resistance Protein (BCRP) efflux transporters (12). Upadacitinib had a relatively short functional half-life of 3 to 4 h following twice-daily administration of the immediate-release formulation; the ER formulation was developed to enable once-daily dosing in patients and is the formulation used in RA phase 3 registrational studies and is the formulation commercially available for adults with RA (13). The efficacy and safety of upadacitinib in patients with moderate to severe RA was evaluated in two phase 2b dose ranging studies and five global phase 3 studies (14,15,16,17,18,19,20). The relationships between upadacitinib plasma exposures and key efficacy and safety endpoints were assessed using data from the phase 2b and 3 clinical trials in RA (21).
A level A non-linear IVIVC was established for upadacitinib ER tablets (8) and accepted by the Food and Drug Administration (FDA) and European Medicines Agency (EMA) (22, 23). In this case study, we describe a model-based approach that was utilized to establish the clinical relevance of the selected dissolution specifications for upadacitinib ER tablet formulation.
MATERIALS AND METHODS
A schematic for the approach utilized to demonstrate the clinical relevance for upadacitinib dissolution specifications is provided in Figure 1. Previously developed IVIVC and exposure-response models for upadacitinib (8, 21) were utilized to conduct pharmacokinetic and exposure-response simulations to evaluate the proposed dissolution specifications for upadacitinib ER formulation.
Use of IVIVC to Predict Upadacitinib Plasma Exposures at the Dissolution Specification Boundaries
A level A IVIVC was developed and validated for upadacitinib ER formulation using Phoenix® Version 7.0 (Pharsight, A Certara® Company, St. Louis, MO, USA) (8). Briefly, the pharmacokinetics of four upadacitinib ER formulations with different in vitro release characteristics and one immediate-release formulation were evaluated in 20 healthy volunteers in a single-dose, randomized, crossover study. The in vivo plasma concentration and in vitro dissolution data were used to establish a non-linear IVIVC, which met the FDA and EMA regulatory validation criteria (8). In vitro dissolution profiles representing the upper and lower boundaries of the dissolution specifications were generated and used as input in the developed IVIVC to predict upadacitinib plasma exposures which correspond to these dissolution limits. The percentage difference between upadacitinib IVIVC-predicted exposures (area under the plasma-concentration time curve [AUC] and maximum plasma concentration [Cmax]) for each of the ER formulation dissolution limits and the predicted exposures for the formulation at the target dissolution profile was calculated.
Upadacitinib Exposure-Response Models
Exposure-response analyses and simulations for upadacitinib efficacy and safety were conducted using NONMEM (Version 7.4.1; Icon, Ellicott City, MD). The exposure-response model development and datasets used are described in detail by Nader et al. (21). Briefly, data from upadacitinib phase 2b and phase 3 studies from 3,685 patients with moderate to severe RA were included in the analyses. The endpoints evaluated in the exposure-response efficacy analyses were American College of Rheumatology (ACR) 20/50/70 and low disease activity (LDA; defined as disease activity score 28-C-reactive protein [DAS28-CRP] ≤ 3.2)/clinical remission (CR; defined as DAS28-CRP < 2.6) endpoints. Exposure-response relationships over 12 to 14 weeks were described by Markov-chain models where upadacitinib plasma concentrations impacted the probability of transitions among the different efficacy states (21).
The relationships between upadacitinib plasma exposures and safety were evaluated using data from 4,577 patients with moderate to severe RA using data from phase 2b and 3 studies (21). Logistic regression models were developed for >2 g/dL decrease from baseline in hemoglobin (at week 12/14 and week 24/26), lymphopenia grade 3 or higher (at week 12/14), and occurrence of any serious infection (during 24 to 26 weeks of treatment); the developed models adequately described the observed exposure-response relationships for upadacitinib safety (21).
Clinical Trial Simulations Using Upadacitinib Efficacy and Safety
Upadacitinib efficacy and safety exposure-response models in RA were used to conduct clinical trial simulations to compare the efficacy and safety of upadacitinib ER formulations which are at the upper and lower boundaries of the dissolution specifications to the target 15 mg formulation, when each of the formulations is administered QD. As shown in Figure 1, the simulated clinical trial included three arms with 300 subjects with moderate to severe RA per arm: (1) upadacitinib 15 mg QD using the target formulation, (2) upadacitinib 15 mg QD using a formulation at the lower dissolution boundary, and (3) upadacitinib 15 mg QD using a formulation at the upper dissolution boundary. For study arms 2 and 3, plasma exposures used in the exposure-response models corresponded to the relative bioavailability predicted from the IVIVC for a formulation at the lower and upper dissolution limits, respectively.
Pharmacokinetic parameters for the simulations for each of the treatment arms were obtained by random sampling with replacement from the empirical Bayesian individual pharmacokinetic parameters for all phase 3 subjects who received upadacitinib (24). Similarly, significant covariates in the efficacy model were included in the simulations by random sampling with replacement from the covariate values for the studied population. For the reference arm, a dose of 15 mg QD was simulated. For the arms simulating the effect of dissolution specifications, the dose amount was adjusted according to the estimated change in the bioavailability for the lower or upper dissolution boundaries based on the IVIVC. The Markov chain exposure-response models for ACR endpoints and LDA/CR were used to perform clinical trial simulations to predict the efficacy of a formulation at the lower dissolution limit (arm 2) compared to the target formulation (arm 1). For each simulation replicate, the difference in model-predicted efficacy responses (percentage of subjects achieving ACR20, ACR50, ACR70, LDA, or CR) between the reference formulation (arm 1) and the formulation at the lower dissolution limit (arm 2) was assessed. Median, 2.5th, and 97.5th percentiles of all differences across the 200 clinical trial replicates were calculated. The noninferiority of arm 2 compared to arm 1 was assessed by comparing the 95% confidence interval for the treatment difference against a noninferiority margin of 10%, which was the margin used in a phase 3 study comparing upadacitinib and adalimumab (25). The lower boundary of the dissolution specifications was considered acceptable if a formulation at this boundary was predicted to meet the noninferiority criteria for the efficacy endpoints compared to the target formulation.
The logistic regression exposure-response models which characterized the relationships between upadacitinib steady-state Cavg and safety were used to conduct clinical trial simulations to support the clinical relevance of the upper dissolution boundary. Clinical trial simulations were conducted using the models to estimate the percentage of subjects treated with the target formulation (arm 1) and a formulation at the upper dissolution limit (arm 3) who are predicted to experience > 2 g/dL decrease from baseline in hemoglobin at week 12, lymphopenia grade 3 or higher at week 12, or a serious infection during 24 weeks of treatment. The median and 90% confidence intervals were calculated across the 200 replicates for each of the evaluated endpoints. The upper dissolution boundary was considered acceptable if a formulation at this boundary was predicted to have no clinically relevant difference in safety events compared to the target formulation.
RESULTS
Clinical trial simulations using the Markov chain efficacy models demonstrated that a formulation that is at the lower dissolution limit is predicted to provide ACR20, ACR50, ACR70, LDA, and CR that is within 2% of the estimated respective responses for the target 15 mg ER formulation. Consistently, a formulation at the lower boundary of the dissolution specifications (arm 2) is predicted to have non-inferior efficacy to the target 15 mg QD formulation (arm 1) with the 95% CI for the difference in efficacy excluding the 10% noninferiority margin (Figure 2).
Non-inferiority efficacy simulation results at week 12 (upadacitinib 15 mg QD tablet at lower dissolution boundary vs. target 15 mg formulation). A ACR response and B LDA/CR response. Dot: median difference in response; error bars: 95% confidence intervals of difference in response; dashed line: 0% difference in response; bold dashed line: non-inferiority margin (M1) representing 10% difference in response
Simulations using the exposure-safety logistic regression models demonstrated that a formulation at the upper boundary of the dissolution specifications is predicted to result in similar percentage of subjects experiencing > 2 g/dL decrease from baseline in hemoglobin at week 12, lymphopenia grade 3 or higher at week 12, or a serious infection during 24 weeks of treatment safety profile to the target 15 mg ER formulation (Figure 3). The selected timepoints for safety simulations correspond to the timepoints for which these endpoints were previously shown to be correlated with upadacitinib plasma exposure.
DISCUSSION
One of the key strengths of modeling and simulation tools is the ability to integrate data across trials and utilize data from prior clinical studies to predict scenarios which have not been evaluated or are not feasible to study. The model-informed drug development paradigm has well-established applications in dose selection, dose optimization, and providing supportive evidence of efficacy to support regulatory decisions (26). In this analysis, we provide a case for applying the model-informed paradigm to support the clinical relevance of dissolution specifications, which is a key aspect in assuring the quality of the drug product (4).
In this analysis, we describe a framework for supporting clinical relevance of modified-release formulations based on coupling IVIVC and exposure-response analysis models for efficacy and safety. This approach can be used to set dissolution specifications based on clinical relevance, regardless of whether the formulations at the upper and lower dissolution boundaries meet the bioequivalence criteria, and can possibly support wider specifications than the traditional approach. A key factor in the success of this approach was the ability to develop a robust and predictive IVIVC (which requires cross-functional collaboration across different disciplines, including clinical pharmacology, biopharmaceutics, and formulation sciences) and exposure-response models (by quantitative clinical pharmacology) to enable linking in vitro dissolution data to efficacy and safety outcomes in the target patient population. As previously described (8), the IVIVC was established using data from an in vivo bioavailability study which was specifically conducted to correlate in vitro dissolution profiles of upadacitinib ER formulations with different release rates with plasma concentrations. The IVIVC robustly met internal and external validation criteria as well as a cross-validation using leave-one-out approach, which enabled the use of the IVIVC model to consistently predict the in vivo pharmacokinetic profiles of upadacitinib ER, which may have in vitro dissolution profiles that are at the upper or lower boundaries of the dissolution specifications. Additionally, the exposure-response models, which correlated upadacitinib plasma concentrations with clinical efficacy and safety endpoints in RA, were established using extensive data across phase 2b and 3 clinical trials in over 3,600 patients with moderate to severe RA and the established models demonstrate adequate predictability of the efficacy and safety in patients with RA (21). Establishing these validated IVIVC and exposure-response models enabled utilization of the models to run simulations which predict the clinical profiles of formulations at the dissolution specification boundaries compared to the target formulation. The simulations supported the clinical relevance of the proposed dissolution specifications given that no relevant difference in safety or efficacy is predicted for lots that are within the proposed acceptance criteria.
In these analyses, we have utilized a statistical noninferiority approach within the simulated clinical trials to evaluate the comparability of efficacy from the formulation at the lower dissolution boundary compared to the target formulation. Noninferiority assessments are commonly used as the primary comparisons in clinical trials which include an active comparator (27). A noninferiority margin of 10% was used in these analyses which is within the range of commonly used margins in actively controlled clinical trials in RA (25, 27). For safety, the relationships between upadacitinib plasma exposures and several key safety endpoints (changes in hemoglobin, serious infections, lymphopenia, and neutropenia) were evaluated. The endpoints/timepoints selected for clinical trial simulations to support the dissolution specifications were the ones which demonstrated correlations with upadacitinib exposure, which were described through the logistic regression analyses.
CONCLUSION
Upadacitinib IVIVC and exposure-response models for efficacy and safety in RA patients were utilized to conduct clinical trial simulations to support the clinical relevance of dissolution specifications for upadacitinib ER tablet formulation. The results demonstrated that a formulation at the lower dissolution boundary is predicted to have noninferior efficacy, and a formulation at the upper dissolution boundary is predicted to have similar safety to the target ER formulation. This case study represents an application for modeling and simulation approaches to support setting clinically relevant dissolution specifications and illustrate the role of modeling and simulations in biopharmaceutics.
References
Suarez-Sharp S, Cohen M, Kesisoglou F, Abend A, Marroum P, Delvadia P, Kotzagiorgis E, Li M, Nordmark A, Bandi N, Sjögren E, Babiskin A, Heimbach T, Kijima S, Mandula H, Raines K, Seo P, Zhang X. Applications of clinically relevant dissolution testing: workshop summary report. AAPS J. 2018;20(6):93.
McAllister M, Flanagan T, Boon K, Pepin X, Tistaert C, Jamei M, et al. Developing clinically relevant dissolution specifications for oral drug products-industrial and regulatory perspectives. Pharmaceutics. 2019;12(1)
Marroum P. Clinically relevant dissolution methods and specifications. Ame Pharmaceu Rev. 2012;
Hermans A, Abend AM, Kesisoglou F, Flanagan T, Cohen MJ, Diaz DA, Mao Y, Zhang L, Webster GK, Lin Y, Hahn DA, Coutant CA, Grady H. Approaches for establishing clinically relevant dissolution specifications for immediate release solid oral dosage forms. AAPS J. 2017;19(6):1537–49.
Paraiso RLM, Rose RH, Fotaki N, McAllister M, Dressman JB. The use of PBPK/PD to establish clinically relevant dissolution specifications for zolpidem immediate release tablets. Eur J Pharm Sci. 2020;155:105534.
AbbVie Inc. RINVOQ™ (upadacitinib extended-release tablets) [US package insert]. North Chicago, IL;. 2019.
AbbVie. Rinvoq (upadacitinib) [summary of product characteristics]. Ludwigshafen, Germany: AbbVie Deutschland GmbH & Co. KG; 2021.
Mohamed MF, Trueman S, Othman AA, Han JH, Ju TR, Marroum P. Development of In vitro-in vivo correlation for upadacitinib extended-release tablet formulation. AAPS J. 2019;21(6):108.
Klunder B, Mittapalli RK, Mohamed MF, Friedel A, Noertersheuser P, Othman AA. Population pharmacokinetics of upadacitinib using the immediate-release and extended-release formulations in healthy subjects and subjects with rheumatoid arthritis: analyses of phase I-III clinical trials. Clin Pharmacokinet. 2019;58(8):1045–58.
Mohamed MF, Camp HS, Jiang P, Padley RJ, Asatryan A, Othman AA. Pharmacokinetics, safety and tolerability of ABT-494, a novel selective JAK 1 inhibitor, in healthy volunteers and subjects with rheumatoid arthritis. Clin Pharmacokinet. 2016;55(12):1547–58.
Mohamed MF, Jungerwirth S, Asatryan A, Jiang P, Othman AA. Assessment of effect of CYP3A inhibition, CYP induction, OATP1B inhibition, and high-fat meal on pharmacokinetics of the JAK1 inhibitor upadacitinib. Br J Clin Pharmacol. 2017;83(10):2242–8.
Mohamed MF, Klunder B, Othman AA. Clinical pharmacokinetics of upadacitinib: review of data relevant to the rheumatoid arthritis indication. Clin Pharmacokinet. 2020;59(5):531–44.
Mohamed MF, Zeng J, Marroum PJ, Song IH, Othman AA. Pharmacokinetics of upadacitinib with the clinical regimens of the extended-release formulation utilized in rheumatoid arthritis phase 3 trials. Clin Pharmacol Drug Dev. 2019;8(2):208–16.
Kremer JM, Emery P, Camp HS, Friedman A, Wang L, Othman AA, et al. A phase IIb study of ABT-494, a selective JAK-1 inhibitor, in patients with rheumatoid arthritis and an inadequate response to anti-tumor necrosis factor therapy. Arthritis Rheumatol. 2016;68(12):2867–77.
Genovese MC, Smolen JS, Weinblatt ME, Burmester GR, Meerwein S, Camp HS, et al. Efficacy and safety of ABT-494, a selective JAK-1 inhibitor, in a phase IIb study in patients with rheumatoid arthritis and an inadequate response to methotrexate. Arthritis Rheumatol. 2016;68(12):2857–66.
Genovese MC, Fleischmann R, Combe B, Hall S, Rubbert-Roth A, Zhang Y, et al. Safety and efficacy of upadacitinib in patients with active rheumatoid arthritis refractory to biologic disease-modifying anti-rheumatic drugs (SELECT-BEYOND): a double-blind, randomised controlled phase 3 trial. Lancet. 2018;391(10139):2513–24. https://doi.org/10.1016/S0140-6736(18)31116-4.
Fleischmann R, Pangan AL, Song IH, Mysler E, Bessette L, Peterfy C, et al. Upadacitinib versus placebo or adalimumab in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase III, double-blind, randomized controlled trial. Arthritis Rheumatol. 2019;71(11):1788–800.
Smolen JS, Pangan AL, Emery P, Rigby W, Tanaka Y, Vargas JI, Zhang Y, Damjanov N, Friedman A, Othman AA, Camp HS, Cohen S. Upadacitinib as monotherapy in patients with active rheumatoid arthritis and inadequate response to methotrexate (SELECT-MONOTHERAPY): a randomised, placebo-controlled, double-blind phase 3 study. Lancet. 2019;393:2303–11.
Burmester GR, Kremer JM, Van den Bosch F, Kivitz A, Bessette L, Li Y, et al. Safety and efficacy of upadacitinib in patients with rheumatoid arthritis and inadequate response to conventional synthetic disease-modifying anti-rheumatic drugs (SELECT-NEXT): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018;391(10139):2503–12.
van Vollenhoven R, Takeuchi T, Pangan AL, Friedman A, Mohamed MF, Chen S, et al. Efficacy and Safety of Upadacitinib Monotherapy in Methotrexate-Naive Patients With Moderately-to-Severely Active Rheumatoid Arthritis (SELECT-EARLY): A multicenter, multi-country, randomized, double-blind, active comparator-controlled trial. Arthrit rheumatol (Hoboken, NJ). 2020;72(10):1607–20.
Nader A, Mohamed MF, Winzenborg I, Doelger E, Noertersheuser P, Pangan AL, et al. Exposure-response analyses of upadacitinib efficacy and safety in phase II and III studies to support benefit-risk assessment in rheumatoid arthritis. Clin Pharmacol Ther. 2020;107(4):994–1003.
Food and Drug Administration, Center for Drug Evaluation and Research. Application Number 211675Orig1s000. Product Quality Review(s) for Upadacitinib Extended Release Tablets. 2019. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/211675Orig1s000Lbl.pdf.
European Medicines Agency. EMA Assessment Report for Rinvoq. EMA/608624/2019 Corr. 1 2019. Available from: https://www.ema.europa.eu/en/documents/assessmentreport/rinvoq-epar-public-assessment-report_en.pdf.
Klunder B, Mohamed MF, Othman AA. Population pharmacokinetics of upadacitinib in healthy subjects and subjects with rheumatoid arthritis: analyses of phase I and II clinical trials. Clin Pharmacokinet. 2018;57(8):977–88.
Fleischmann RM, Genovese MC, Enejosa JV, Mysler E, Bessette L, Peterfy C, Durez P, Ostor A, Li Y, Song IH. Safety and effectiveness of upadacitinib or adalimumab plus methotrexate in patients with rheumatoid arthritis over 48 weeks with switch to alternate therapy in patients with insufficient response. Ann Rheum Dis. 2019;78(11):1454–62.
Wang Y, Zhu H, Madabushi R, Liu Q, Huang SM, Zineh I. Model-informed drug development: current US regulatory practice and future considerations. Clin Pharmacol Ther. 2019;105(4):899–911.
Rothwell R, Nikolov NP, Maynard JW, Levin G. Noninferiority trials to evaluate drug effects in rheumatoid arthritis. Arthrit rheumatol (Hoboken, NJ). 2020;72(8):1258–65.
Acknowledgements
The authors thank Eva Doelger, an employee of AbbVie, for conducting the exposure-safety simulations. Medical writing support was provided by Mia DeFino, MS, ELS, a freelance medical writer under contract with AbbVie.
Data Sharing
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial-level data (analysis data sets), as well as other information (e.g., protocols and Clinical Study Reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. This clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time, and the data will be accessible for 12 months, with possible extensions considered. For more information on the process or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.
Funding
AbbVie provided financial support for the studies and participated in the study design, study conduct, and analysis and interpretation of data and the writing, review, and approval of the manuscript.
Author information
Authors and Affiliations
Contributions
All authors provided substantial contributions to the study design, data acquisition and analysis, and interpretation of the data. All authors participated in the drafting and revising of the manuscript and approve its final form for publication.
Corresponding author
Ethics declarations
Conflict of interest
Mohamed-Eslam F. Mohamed, Insa Winzenborg, and Patrick Marroum are employees of AbbVie and may hold AbbVie stock. Ahmed A. Othman is a former employee of AbbVie and may hold AbbVie stock.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Affiliation at the time this research was conducted
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mohamed, ME.F., Winzenborg, I., Othman, A.A. et al. Utility of Modeling and Simulation Approach to Support the Clinical Relevance of Dissolution Specifications: a Case Study from Upadacitinib Development. AAPS J 24, 39 (2022). https://doi.org/10.1208/s12248-022-00681-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-022-00681-6