Abstract
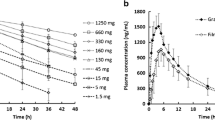
In vivo drug dissolution kinetics of BCS Class 2a IR solid oral drug products remains largely unknown. An understanding to what extent the solubility influences in vivo dissolution is needed to design appropriate in vitro dissolution methods. In this study, nonsteroidal anti-inflammatory drugs (NSAIDs) are used to investigate the in vivo dissolution of BCS Class 2a drugs based on numerical deconvolution analyses. The PK data were obtained from published literature or drug applications submitted to the FDA. It has been hypothesized that the in vivo drug dissolution rate would likely correlate to the solubility of NSAIDs in the media at gastrointestinal pH. Our findings show a short lag time of absorption (Tlag), comparable to the liquid gastric emptying time and independent of the solubility and formulation. In Vivo drug dissolution of NSAIDs was generally rapid and complete within the regular drug residence time in the small intestine while multi-phase absorption was observed in some subjects for all the NSAIDs. The comparisons of in vivo drug dissolution rate, which was characterized by in vivo dissolution half-life (Thalf), indicate that solubility has a minimal impact on in vivo drug dissolution rate for NSAIDs. Gastric emptying regulated by migrating motor complex (MMC) under fasted state most likely governs drug dissolution and absorption of NSAIDs. For BCS Class 2a IR solid oral drug products, large variability of gastric emptying and MMC as well as the strong driving force of intestinal absorption probably outweigh the impact of solubility on drug in vivo dissolution.
Graphic Abstract




Similar content being viewed by others
References
ICH Harmonised Guideline. Biopharmaceutics classification system-based biowaivers M9. Int Counc Harmon Tech Requir Pharm Hum Use. 2020.
Tsume Y, Mudie DM, Langguth P, Amidon GE, Amidon GL. The Biopharmaceutics Classification System: subclasses for in vivo predictive dissolution (IPD) methodology and IVIVC. Eur J Pharm Sci. 2014;57:152–63. https://doi.org/10.1016/j.ejps.2014.01.009.
DeSesso JM, Jacobson CF. Anatomical and physiological parameters affecting gastrointestinal absorption in humans and rats. Food Chem Toxicol. 2001;39(3):209–28. https://doi.org/10.1016/s0278-6915(00)00136-8.
Yazdanian M, Briggs K, Jankovsky C, Hawi A. The, “high solubility” definition of the current FDA Guidance on Biopharmaceutical Classification System may be too strict for acidic drugs. Pharm Res. 2004;21(2):293–9. https://doi.org/10.1023/b:pham.0000016242.48642.71.
Van Den Abeele J, Brouwers J, Mattheus R, Tack J, Augustijns P. Gastrointestinal behavior of weakly acidic BCS Class II drugs in man—case study of diclofenac potassium. J Pharm Sci. 2016;105(2):687–96. https://doi.org/10.1002/jps.24647.
Koenigsknecht MJ, Baker JR, Wen B, Frances A, Zhang H, Yu A, et al. In vivo dissolution and systemic absorption of immediate release ibuprofen in human gastrointestinal tract under fed and fasted conditions. Mol Pharm. 2017;14(12):4295–304. https://doi.org/10.1021/acs.molpharmaceut.7b00425.
Bermejo M, Paixao P, Hens B, Tsume Y, Koenigsknecht MJ, Baker JR, et al. Linking the gastrointestinal behavior of ibuprofen with the systemic exposure between and within humans-part 1: fasted state conditions. Mol Pharm. 2018;15(12):5454–67. https://doi.org/10.1021/acs.molpharmaceut.8b00515.
Yu A, Koenigsknecht MJ, Hens B, Baker JR, Wen B, Jackson TL, et al. Mechanistic deconvolution of oral absorption model with dynamic gastrointestinal fluid to predict regional rate and extent of GI drug dissolution. AAPS J. 2019;22(1):3. https://doi.org/10.1208/s12248-019-0385-z.
Sjogren E, Westergren J, Grant I, Hanisch G, Lindfors L, Lennernas H, et al. In silico predictions of gastrointestinal drug absorption in pharmaceutical product development: application of the mechanistic absorption model GI-Sim. Eur J Pharm Sci. 2013;49(4):679–98. https://doi.org/10.1016/j.ejps.2013.05.019.
Tsume Y, Langguth P, Garcia-Arieta A, Amidon GL. In silico prediction of drug dissolution and absorption with variation in intestinal pH for BCS class II weak acid drugs: ibuprofen and ketoprofen. Biopharm Drug Dispos. 2012;33(7):366–77. https://doi.org/10.1002/bdd.1800.
Pavliv L, Voss B, Rock A. Pharmacokinetics, safety, and tolerability of a rapid infusion of i.v. ibuprofen in healthy adults. Am J Health Syst Pharm. 2011;68(1):47–51. https://doi.org/10.2146/ajhp100120.
Jensen KM, Grenabo L. Bioavailability of indomethacin after intramuscular injection and rectal administration of solution and suppositories. Acta Pharmacol Toxicol (Copenh). 1985;57(5):322–7. https://doi.org/10.1111/j.1600-0773.1985.tb00052.x.
Veng-Pedersen P, Modi NB. An Algorithm for constrained deconvolution based on reparameterization. J Pharm Sci. 1992;81(2):175–80. https://doi.org/10.1002/jps.2600810214.
Fallingborg J. Intraluminal pH of the human gastrointestinal tract. Dan Med Bull. 1999;46(3):183–96.
Turck D, Roth W, Busch U. A review of the clinical pharmacokinetics of meloxicam. Br J Rheumatol. 1996;35(Suppl 1):13–6. https://doi.org/10.1093/rheumatology/35.suppl_1.13.
Willis JV, Kendall MJ, Flinn RM, Thornhill DP, Welling PG. The pharmacokinetics of diclofenac sodium following intravenous and oral administration. Eur J Clin Pharmacol. 1979;16(6):405–10. https://doi.org/10.1007/bf00568201.
Oberle RL, Amidon GL. The influence of variable gastric emptying and intestinal transit rates on the plasma level curve of cimetidine; an explanation for the double peak phenomenon. J Pharmacokinet Biopharm. 1987;15(5):529–44. https://doi.org/10.1007/BF01061761.
Hunt JN, Macdonald I. The influence of volume on gastric emptying. J Physiol. 1954;126(3):459–74. https://doi.org/10.1113/jphysiol.1954.sp005222.
Bateman DN, Whittingham TA. Measurement of gastric emptying by real-time ultrasound. Gut. 1982;23(6):524–7. https://doi.org/10.1136/gut.23.6.524.
Braghetto I, Davanzo C, Korn O, Csendes A, Valladares H, Herrera E, et al. Scintigraphic evaluation of gastric emptying in obese patients submitted to sleeve gastrectomy compared to normal subjects. Obes Surg. 2009;19(11):1515–21. https://doi.org/10.1007/s11695-009-9954-z.
Ramsbottom N, Knox MT, Hunt JN. Gastric emptying of barium sulphate suspension compared with that of water. Gut. 1977;18(7):541–2. https://doi.org/10.1136/gut.18.7.541.
Umenai T, Arai N, Chihara E. Effect of the preliminary hydration on gastric emptying time for water in healthy volunteers. Acta Anaesthesiol Scand. 2009;53(2):223–6. https://doi.org/10.1111/j.1399-6576.2008.01832.x.
Paixão P, Bermejo M, Hens B, Tsume Y, Dickens J, Shedden K, et al. Gastric emptying and intestinal appearance of nonabsorbable drugs phenol red and paromomycin in human subjects: a multi-compartment stomach approach. Eur J Pharm Biopharm. 2018;129:162–74. https://doi.org/10.1016/j.ejpb.2018.05.033.
Rhie JK, Hayashi Y, Welage LS, Frens J, Wald RJ, Barnett JL, et al. Drug marker absorption in relation to pellet size, gastric motility and viscous meals in humans. Pharm Res. 1998;15(2):233–8. https://doi.org/10.1023/a:1011962501270.
Mudie DM, Murray K, Hoad CL, Pritchard SE, Garnett MC, Amidon GL, et al. Quantification of gastrointestinal liquid volumes and distribution following a 240 mL dose of water in the fasted state. Mol Pharm. 2014;11(9):3039–47. https://doi.org/10.1021/mp500210c.
FDA Guidance for Industry: Bioavailability and Bioequivalence Studies Submitted in NDAs or INDs — General Considerations. United States Food and Drug Administration. 2014.
Gupta A. Recent trends of fast dissolving tablet - an overview of formulation technology. Int J Pharma Biol Archive. 2010;1(1):1–10.
Hens B, Tsume Y, Bermejo M, Paixao P, Koenigsknecht MJ, Baker JR, et al. Low buffer capacity and alternating motility along the human gastrointestinal tract: implications for in vivo dissolution and absorption of ionizable drugs. Mol Pharm. 2017;14(12):4281–94. https://doi.org/10.1021/acs.molpharmaceut.7b00426.
Kambayashi A, Sako K, Kondo H. Characterization of the buccal and gastric transit of orally disintegrating tablets in humans using gamma scintigraphy. Int J Pharm. 2020;576: 118937. https://doi.org/10.1016/j.ijpharm.2019.118937.
Litou C, Vertzoni M, Goumas C, Vasdekis V, Xu W, Kesisoglou F, et al. Characteristics of the Human upper gastrointestinal contents in the fasted state under hypo- and A-chlorhydric gastric conditions under conditions of typical drug - drug interaction studies. Pharm Res. 2016;33(6):1399–412. https://doi.org/10.1007/s11095-016-1882-8.
Clements JA, Heading RC, Nimmo WS, Prescott LF. Kinetics of acetaminophen absorption and gastric emptying in man. Clin Pharmacol Ther. 1978;24(4):420–31. https://doi.org/10.1002/cpt1978244420.
Feher J. 8.3 - Intestinal and Colonic Chemoreception and Motility. In: Feher J, editor. Quantitative Human Physiology (Second Edition). Academic Press; 2017. p. 796–809.
Dooley CP, Di Lorenzo C, Valenzuela JE. Variability of migrating motor complex in humans. Dig Dis Sci. 1992;37(5):723–8. https://doi.org/10.1007/BF01296429.
Higaki K, Choe SY, Lobenberg R, Welage LS, Amidon GL. Mechanistic understanding of time-dependent oral absorption based on gastric motor activity in humans. Eur J Pharm Biopharm. 2008;70(1):313–25. https://doi.org/10.1016/j.ejpb.2008.02.022.
Meyer JH, Elashoff J, Porter-Fink V, Dressman J, Amidon GL. Human postprandial gastric emptying of 1–3-millimeter spheres. Gastroenterology. 1988;94(6):1315–25. https://doi.org/10.1016/0016-5085(88)90669-5.
Lipka E, Lee ID, Langguth P, Spahn-Langguth H, Mutschler E, Amidon GL. Celiprolol double-peak occurrence and gastric motility: nonlinear mixed effects modeling of bioavailability data obtained in dogs. J Pharmacokinet Biopharm. 1995;23(3):267–86. https://doi.org/10.1007/BF02354285.
Van Den Abeele J, Schilderink R, Schneider F, Mols R, Minekus M, Weitschies W, et al. Gastrointestinal and systemic disposition of diclofenac under fasted and fed state conditions supporting the evaluation of in vitro predictive tools. Mol Pharm. 2017;14(12):4220–32. https://doi.org/10.1021/acs.molpharmaceut.7b00253.
Abuhelwa AY, Foster DJR, Upton RN. A Quantitative review and meta-models of the variability and factors affecting oral drug absorption-Part II: gastrointestinal transit time. AAPS J. 2016;18(5):1322–33. https://doi.org/10.1208/s12248-016-9953-7.
Yuen KH. The transit of dosage forms through the small intestine. Int J Pharm. 2010;395(1–2):9–16. https://doi.org/10.1016/j.ijpharm.2010.04.045.
Davis SS, Hardy JG, Fara JW. Transit of pharmaceutical dosage forms through the small intestine. Gut. 1986;27(8):886–92. https://doi.org/10.1136/gut.27.8.886.
Ibekwe VC, Fadda HM, McConnell EL, Khela MK, Evans DF, Basit AW. Interplay between intestinal pH, transit time and feed status on the in vivo performance of pH responsive ileo-colonic release systems. Pharm Res. 2008;25(8):1828–35. https://doi.org/10.1007/s11095-008-9580-9.
Lee KJ, Vos R, Janssens J, Tack J. Influence of duodenal acidification on the sensorimotor function of the proximal stomach in humans. Am J Physiol Gastrointest Liver Physiol. 2004;286(2):G278–84. https://doi.org/10.1152/ajpgi.00086.2003.
Madsen JL, Krogsgaard OW. Gastrointestinal scintiscanning: dosimetry. Eur J Nucl Med. 1989;15(5):260–1. https://doi.org/10.1007/BF00257544.
Perez de la Cruz Moreno M, Oth M, Deferme S, Lammert F, Tack J, Dressman J. Characterization of fasted-state human intestinal fluids collected from duodenum and jejunum. J Pharm Pharmacol. 2006;58(8):1079–89. https://doi.org/10.1211/jpp.58.8.0009.
Krieg BJ, Taghavi SM, Amidon GL, Amidon GE. In vivo predictive dissolution: transport analysis of the CO2, bicarbonate in vivo buffer system. J Pharm Sci. 2014;103(11):3473–90. https://doi.org/10.1002/jps.24108.
Al-Gousous J, Sun KX, McNamara DP, Hens B, Salehi N, Langguth P, Bermejo M, Amidon GE, Amidon GL. Mass transport analysis of the enhanced buffer capacity of the bicarbonate-CO2 buffer in a phase-heterogenous system: physiological and pharmaceutical significance. Mol Pharm. 2018;15(11):5291–301. https://doi.org/10.1021/acs.molpharmaceut.8b00783.
Al-Gousous J, Salehi N, Amidon GE, Ziff RM, Langguth P, Amidon GL. Mass transport analysis of bicarbonate buffer: effect of the CO2-H2CO3 hydration-dehydration kinetics in the fluid boundary layer and the apparent effective p Ka controlling dissolution of acids and bases. Mol Pharm. 2019;16(6):2626–35. https://doi.org/10.1021/acs.molpharmaceut.9b00187.
Dalenbäck J, Fändriks L, Olbe L, Sjövall H. Mechanisms behind changes in gastric acid and bicarbonate outputs during the human interdigestive motility cycle. Am J Physiol. 1996 Jan;270(1 Pt 1):G113–22. https://doi.org/10.1152/ajpgi.1996.270.1.G113.
Yu LX, Lipka E, Crison JR, Amidon GL. Transport approaches to the biopharmaceutical design of oral drug delivery systems: prediction of intestinal absorption. Adv Drug Deliv Rev. 1996;19(3):359–76. https://doi.org/10.1016/0169-409x(96)00009-9.
Funding
Drs Zhang and Wu were supported in part by the appointments to the Research Participation Program at the Center for Drug Evaluation and Research, administered by the Oak Ridge Institute for Science and Education.
Author information
Authors and Affiliations
Contributions
All authors were involved in the conceptualization, methodology, data analyses, interpretation and in the development of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The findings and conclusions in this work have not been formally disseminated by the United States Food and Drug Administration and should not be construed to represent any Agency determination or policy.
Additional information
Guest Editors: Rodrigo Cristofoletti and Lawrence Yu
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, M., Zhang, X., Wu, D. et al. Understanding In Vivo Dissolution of Immediate Release (IR) Solid Oral Drug Products Containing Weak Acid BCS Class 2 (BCS Class 2a) Drugs. AAPS J 23, 113 (2021). https://doi.org/10.1208/s12248-021-00639-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-021-00639-0