Abstract
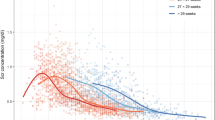
Serum creatinine (sCr) is a commonly measured biomarker to estimate glomerular filtration rate (GFR) and therefore widely used as a covariate in population pharmacokinetic models of renally excreted drugs. In neonates, sCr dynamically changes during the first few weeks after birth. Missing covariates are a common problem in pharmacokinetic modeling of neonates due to the limited availability of blood sampling in number and volume. The objective of this work is to develop a parsimonious population model describing time courses of sCr in neonates with the intent to be incorporated into pharmacokinetic models of various drugs where sCr values are sparse or missing. The data for model development consisted of sCr measurements in 1080 newborns with a gestational age of 24–42 weeks. The model is based on a pharmacokinetic model of sCr that involves GFR, backflow of creatinine from the renal tubules, and urinary flow. Gestational age is the only covariate explaining between-subject variability of sCr. The model adequately describes distinct features of the sCr time course such as a peak and decline to a plateau. For a neonate with a GA of 35 weeks, the typical value of sCr at birth was 0.584 mg/dL, the peak (0.794 mg/dL) occurred 2.3 days after birth, to reach a plateau of 0.255 mg/dL approximately after 24.7 days. Model simulations reveal that in neonates with a similar postnatal age, sCr decreases with increasing GA. In summary, our model is designed to be a part of full random effects pharmacokinetic models where sCr is a significant covariate.








Similar content being viewed by others
References
Food and Drug Administration. General Clinical Pharmacology Considerations for Neonatal Studies for Drugs and Biological Products Guidance for Industry. 2019. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/general-clinical-pharmacology-considerations-neonatal-studies-drugs-and-biological-products-guidance Accessed December 2020.
Ward RM, Benjamin D, Barrett JS, Allegaert K, Portman R, Davis JM, et al. Safety, dosing, and pharmaceutical quality for studies that evaluate medicinal products (including biological products) in neonates. Pediatr Res. 2017;81:692–711. https://doi.org/10.1038/pr.2016.221.
Guignard JP, Drukker A. Why do newborn infants have a high plasma creatinine? Pediatrics. 1999;103:e49.
Gubhaju L, Sutherland MR, Horne RS, Medhurst A, Kent AL, Ramsden A, et al. Assessment of renal functional maturation and injury in preterm neonates during the first month of life. Am J Physiol Renal Physiol. 2014;307:F149–58. https://doi.org/10.1152/ajprenal.00439.2013.
Grimsley C, Thomson AH. Pharmacokinetics and dose requirements of vancomycin in neonates. Arch Dis Child Fetal Neonatal Ed. 1999;81:F221–7.
Anderson BJ, Allegaert K, Holford NH. Population clinical pharmacology of children: modelling covariate effect. Eur J Pediatr. 2006;165:819–29. https://doi.org/10.1007/s00431-006-0189-x.
Nyberg J, Karlsson MO, Jonsson EN. Implicit and efficient handling of missing covariate information using full random effects modelling. PAGE 28 (2019) Abstr 9181. www.page-meeting.org/?abstract=9181. Accessed Jan 2021.
van Donge T, Allegaert K, Gotta V, Smits A, Levtchenko E, Mekahli D, et al. Characterizing dynamics of serum creatinine and creatinine clearance in extremely low birth weight neonates during the first 6 weeks of life. Pediatric Nephrology. 2021;36:649–59. https://doi.org/10.1007/s00467-020-04749-3.
Allegaert K. Creatinine Assays in Early Infancy: How to Aim for a Moving Target. In: Patel VB, Preedy VR, editors. Biomarkers in Kidney Disease. Dordrecht: Springer Science+Business Media; 2015.
Delanghe JR, Cobbaert C, Harmoinen A, Jansen R, Laitinen P, Panteghini M. Focusing on the clinical impact of standardization of creatinine measurements: a report by the EFCC Working Group oncreatinine standardization. Clin Chem Lab Med. 2011;49:977–82.
Allegaert K, Pauwels S, Smits A, Crevecoeur K, van den Anker J, Mekahli D, et al. Enzymatic isotope dilution mass spectrometry (IDMS) traceable serum creatinine is preferable over Jaffe in neonates and young infants. Clin Chem Lab Med. 2014;52:e107–9.
Erlandsen EJ, Randers E. Challenges in the measurement of plasma creatinine on the Roche cobas c702. Scand J Clin Lab Invest. 2018;78:490–5. https://doi.org/10.1080/00365513.2018.1501090.
Felmlee MA, Dave RA, Morris ME. Mechanistic models describing active renal reabsorption and secretion: a simulation-based study. AAPS J. 2012;15:278–87. https://doi.org/10.1208/s12248-012-9437-3.
Gabrielsson J, Weiner D. Pharmacokinetic and pharmacodynamic data analysis: concepts and applications. 4th ed. Stockholm: Swedish Pharmaceutical Society; 2007.
Janzen DLI, Bergenholm L, Jirstrand M, Parkinson J, Yates J, Evans ND, et al. Parameter identifiability of fundamental pharmacodynamic models. Front. Physiol. 2016;7:590. https://doi.org/10.3389/fphys.2016.00590.
Bonate PL. Pharmacokinetic-pharmacodynamic modeling and simulation. 2nd ed. New York: Springer Science; 2011.
Yano Y, Beal SL, Sheiner LB. Evaluating pharmacokinetic/pharmacodynamic models using the posterior predictive check. J Pharmacokinet Pharmacodyn. 2001;28:171–92.
The R Project for Statistical Computing. https://www.r-project.org/. Accessed 28 June 2020.
Ceriotti F, Boyd JC, Klein G, Henny J, Queralto J, Kairisto V, et al. Reference intervals for serum creatinine concentrations: assessment of available data for global application. Clinical Chemistry. 2008;54(3):559–66.
Boer DP, de Rijke YB, Hop WC, Cransberg K, Dorresteijn EM. Reference values for serum creatinine in children younger than 1 year of age. Pediatr Nephrol. 2010;25:2107–13. https://doi.org/10.1007/s00467-010-1533-y.
Farrell PC, Grib NL, Fry DL, Popovich RP, Broviac JW, Babb AL. A comparison of in vitro and in vivo solute-protein binding interactions in normal and uremic subjects. Trans Amer Soc Artf Int Organs. 1972;18:268–81.
Brion LP, Fleischman AR, McCarton C, Schwartz GJ. A simple estimate of glomerular filtration rate in low birth weight infants during the first year of life: noninvasive assessment of body composition and growth. J Pediatr. 1986;109:698–707.
Go H, Momoi N, Kashiwabara N, Haneda K, Chishiki M, Imamura T, et al. Neonatal and maternal serum creatinine levels during the early postnatal period in preterm and term infants. PLoS ONE. 2018;13:e0196721. https://doi.org/10.1371/journal.pone.0196721.
Savory DJ. Reference ranges for serum creatinine in infants, children and adolescents. Ann Clin Biochem. 1990;27:99–101.
Johansson AM, Karlsson MO. Comparison of methods for handling missing covariate data. AAPS J. 2013;15:1232–41.
Krzyzanski W, Cook SF, Wilbaux M, Sherwin CMT, Allegaert K, Vermeulen A, et al. Population pharmacokinetic modeling in the presence of missing time-dependent covariates: Impact of body weight on pharmacokinetics of paracetamol in neonates. AAPS J. 2019;21:68. https://doi.org/10.1208/s12248-019-0331-0.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 2776 kb)
Appendix. Derivation of the quasi-steady state Eq. (6)
Appendix. Derivation of the quasi-steady state Eq. (6)
Dividing Eqs. (3)–(4) by GFR0, one can obtain the time scale for creatinine clearance Vp/GFR0 that is equal to 0.3 days for a typical term neonate of BWT =2.5 kg. If the time scale for GFR(t) due to kidney maturation is several days or longer, the factor Vp/GFR0 will be proportionally decreased, justifying the quasi-steady-state assumption Eq. (5). Consequently,
One can add Eqs. (16) and (17) side by side and arrive at
Hence
On the other hand, solving Eq. (17) for yields
Substituting rtCr from Eq. (19) into Eq. (20) results in Eq. (6).
Rights and permissions
About this article
Cite this article
Krzyzanski, W., Smits, A., Van Den Anker, J. et al. Population Model of Serum Creatinine as Time-Dependent Covariate in Neonates. AAPS J 23, 86 (2021). https://doi.org/10.1208/s12248-021-00612-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-021-00612-x