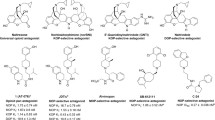
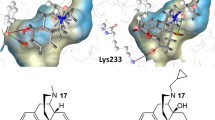
Abstract
The nociceptin opioid receptor (NOP), the fourth member of the opioid receptor family, and its endogenous peptide ligand, nociceptin or orphanin FQ (N/OFQ), play a vital role in several central nervous system pathways regulating pain, reward, feeding, anxiety, motor control and learning/memory. Both selective NOP agonists as well as bifunctional agonists at the NOP and mu opioid receptor (MOP) have potential therapeutic applications in CNS disorders related to these processes. Using Surflex-Dock protocols, we conducted a computational structure-activity study of four scaffold classes of NOP ligands with varying NOP-MOP selectivity. By docking these compounds into the orthosteric binding sites within an active-state NOP homology model, and an active-state MOP crystal structure, the goal of this study was to use a structure-based drug design approach to modulate NOP affinity and NOP vs. MOP selectivity. We first docked four parent compounds (no side chain) to determine their binding interactions within the NOP and MOP binding pockets. Various polar sidechains were added to the heterocyclic A-pharmacophore to modulate NOP ligand affinity. The substitutions mainly contained a 1-2 carbon chain with a polar substituent such as an amine, alcohol, sulfamide, or guanidine. The SAR analysis is focused on the impact of structural changes in the sidechain, such as chain length, hydrogen bonding capability, and basic vs neutral functional groups on binding affinity and selectivity at both NOP and MOP receptors. This study highlights structural modifications that can be leveraged to rationally design both selective NOP and bifunctional NOP-MOP agonists with different ratios of functional efficacy.
Graphical abstract






Similar content being viewed by others
References
Bunzow JR, Saez C, Mortrud M, Bouvier C, Williams JT, Low M, et al. Molecular cloning and tissue distribution of a putative member of the rat opioid receptor gene family that is not a mu, delta or kappa opioid receptor type. FEBS Lett. 1994;347(2-3):284–8.
Mollereau C, Parmentier M, Mailleux P, Butour JL, Moisand C, Chalon P, et al. ORL1, a novel member of the opioid receptor family. Cloning, functional expression and localization. FEBS Lett. 1994;341(1):33–8.
Fukuda K, Kato S, Mori K, Nishi M, Takeshima H, Iwabe N, et al. cDNA cloning and regional distribution of a novel member of the opioid receptor family. FEBS Lett. 1994;343(1):42–6.
Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, et al. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377(6549):532–5.
Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, et al. Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science. 1995;270(5237):792–4.
Chen Y, Fan Y, Liu J, Mestek A, Tian M, Kozak CA, et al. Molecular cloning, tissue distribution and chromosomal localization of a novel member of the opioid receptor gene family. FEBS Lett. 1994;347(2-3):279–83.
Narita M, Mizoguchi H, Oji DE, Dun NJ, Hwang BH, Nagase H, et al. Identification of the G-protein-coupled ORL1 receptor in the mouse spinal cord by [35S]-GTPgammaS binding and immunohistochemistry. Br J Pharmacol. 1999;128(6):1300–6.
Sandin J, Georgieva J, Schott PA, Ogren SO, Terenius L. Nociceptin/orphanin FQ microinjected into hippocampus impairs spatial learning in rats. Eur J Neurosci. 1997;9(1):194–7.
Andero R. Nociceptin and the nociceptin receptor in learning and memory. Prog Neuro-Psychopharmacol Biol Psychiatry. 2015;62:45–50.
Jenck F, Moreau JL, Martin JR, Kilpatrick GJ, Reinscheid RK, Monsma FJ Jr, et al. Orphanin FQ acts as an anxiolytic to attenuate behavioral responses to stress. Proc Natl Acad Sci U S A. 1997;94(26):14854–8.
Gavioli EC, de Medeiros IU, Monteiro MC, Calo G, Romao PR. Nociceptin/orphanin FQ-NOP receptor system in inflammatory and immune-mediated diseases. Vitam Horm. 2015;97:241–66.
Kiguchi N, Ding H, Kishioka S, Ko MC. Nociceptin/Orphanin FQ Peptide Receptor-Related Ligands as Novel Analgesics. Curr Top Med Chem. 2020;20(31):2878–88.
Mercatelli D, Pisano CA, Novello S, Morari M. NOP receptor ligands and Parkinson's disease. Handb Exp Pharmacol. 2019;254:213–32.
Ciccocioppo R, Borruto AM, Domi A, Teshima K, Cannella N, Weiss F. NOP-Related Mechanisms in Substance Use Disorders. Handb Exp Pharmacol. 2019;254:187–212.
Mouledous L. The nociceptin/orphanin FQ system and the regulation of memory. Handb Exp Pharmacol. 2019;254:259–78.
Kiguchi N, Ding H, Ko MC. Therapeutic potentials of NOP and MOP receptor coactivation for the treatment of pain and opioid abuse. J Neurosci Res. 2020.
Zaveri NT. Nociceptin Opioid Receptor (NOP) as a Therapeutic Target: Progress in Translation from Preclinical Research to Clinical Utility. J Med Chem. 2016;59(15):7011–28.
Witkin JM, Wallace TL, Martin WJ. Therapeutic approaches for NOP receptor antagonists in neurobehavioral disorders: clinical studies in major depressive disorder and alcohol use disorder with BTRX-246040 (LY2940094). Handb Exp Pharmacol. 2019.
Woodcock A, McLeod RL, Sadeh J, Smith JA. The efficacy of a NOP1 agonist (SCH486757) in subacute cough. Lung. 2010;188(Suppl 1):S47–52.
Eerdekens MH, Kapanadze S, Koch ED, Kralidis G, Volkers G, Ahmedzai SH, et al. Cancer-related chronic pain: investigation of the novel analgesic drug candidate cebranopadol in a randomized, double-blind, noninferiority trial. European journal of pain (London, England). 2018.
Scholz A, Bothmer J, Kok M, Hoschen K, Daniels S. Cebranopadol: a novel, first-in-class, strong analgesic: results from a randomized phase IIA clinical trial in postoperative acute Pain. Pain Phys. 2018;21(3):E193–e206.
Di Giannuario A, Pieretti S, Catalani A, Loizzo A. Orphanin FQ reduces morphine-induced dopamine release in the nucleus accumbens: a microdialysis study in rats. Neurosci Lett. 1999;272(3):183–6.
Murphy NP, Ly HT, Maidment NT. Intracerebroventricular orphanin FQ/nociceptin suppresses dopamine release in the nucleus accumbens of anaesthetized rats. Neuroscience. 1996;75(1):1–4.
Cremeans CM, Gruley E, Kyle DJ, Ko MC. Roles of mu-opioid receptors and nociceptin/orphanin FQ peptide receptors in buprenorphine-induced physiological responses in primates. J Pharmacol Exp Ther. 2012;343(1):72–81.
Hu E, Calo G, Guerrini R, Ko MC. Long-lasting antinociceptive spinal effects in primates of the novel nociceptin/orphanin FQ receptor agonist UFP-112. Pain. 2010;148(1):107–13.
Khroyan TV, Zaveri NT, Polgar WE, Orduna J, Olsen C, Jiang F, et al. SR 16435 [1-(1-(bicyclo[3.3.1]nonan-9-yl)piperidin-4-yl)indolin-2-one], a novel mixed nociceptin/orphanin FQ/mu-opioid receptor partial agonist: analgesic and rewarding properties in mice. J Pharmacol Exp Ther. 2007;320(2):934–43.
Toll L, Khroyan TV, Polgar WE, Jiang F, Olsen C, Zaveri NT. Comparison of the antinociceptive and antirewarding profiles of novel bifunctional nociceptin receptor/mu-opioid receptor ligands: implications for therapeutic applications. J Pharmacol Exp Ther. 2009;331(3):954–64.
Ding H, Kiguchi N, Yasuda D, Daga PR, Polgar WE, Lu JJ, et al. A bifunctional nociceptin and mu opioid receptor agonist is analgesic without opioid side effects in nonhuman primates. Sci Transl Med. 2018;10(456).
Schunk S, Linz K, Hinze C, Frormann S, Oberbörsch S, Sundermann B, et al. Discovery of a potent analgesic NOP and opioid receptor agonist: cebranopadol. ACS Med Chem Lett. 2014;5(8):857–62.
Linz K, Christoph T, Tzschentke TM, Koch T, Schiene K, Gautrois M, et al. Cebranopadol: a novel potent analgesic nociceptin/orphanin FQ peptide and opioid receptor agonist. J Pharmacol Exp Ther. 2014;349(3):535–48.
Cami-Kobeci G, Polgar WE, Khroyan TV, Toll L, Husbands SM. Structural determinants of opioid and NOP receptor activity in derivatives of buprenorphine. J Med Chem. 2011;54(19):6531–7.
Kiguchi N, Ding H, Cami-Kobeci G, Sukhtankar DD, Czoty PW, DeLoid HB, et al. BU10038 as a safe opioid analgesic with fewer side-effects after systemic and intrathecal administration in primates. Br J Anaesth. 2019;122(6):e146–e56.
Zaveri NT, Jiang F, Olsen C, Polgar WE, Toll L. Designing bifunctional NOP receptor-mu opioid receptor ligands from NOP receptor-selective scaffolds. Part 1. Bioorg Med Chem Lett. 2013;23(11):3308–13.
Journigan VB, Polgar WE, Khroyan TV, Zaveri NT. Designing bifunctional NOP receptor-mu opioid receptor ligands from NOP-receptor selective scaffolds. Part II. Bioorg Med Chem. 2014;22(8):2508–16.
Zaveri NT, Yasuda D, Journigan VB, Daga PD, Jiang F, Olsen C. Structure-activity relationships of Nociceptin Receptor (NOP) Ligands and the Design of Bifunctional NOP/mu opioid receptor-targeted Ligands. In: Ko MC, Husbands SM, editors. Research and Development of Opioid-Related Analgesics: American Chemical Society; 2013. p. 145–60.
Zaveri NT, Meyer ME. NOP-Targeted Nonpeptide Ligands. Handb Exp Pharmacol. 2019;254:37–67.
Kamakolanu UG, Meyer ME, Yasuda D, Polgar WE, Marti M, Mercatelli D, et al. Discovery and structure-activity relationships of nociceptin receptor partial agonists that afford symptom ablation in Parkinson's disease models. J Med Chem. 2020;63(5):2688–704.
Daga PR, Zaveri NT. Homology modeling and molecular dynamics simulations of the active state of the nociceptin receptor reveal new insights into agonist binding and activation. Proteins. 2012;80(8):1948–61.
Zaveri N, Jiang F, Olsen C, Polgar W, Toll L. Small-molecule agonists and antagonists of the opioid receptor-like receptor (ORL1, NOP): ligand-based analysis of structural factors influencing intrinsic activity at NOP. AAPS J. 2005;7(2):E345–52.
Meng F, Taylor LP, Hoversten MT, Ueda Y, Ardati A, Reinscheid RK, et al. Moving from the orphanin FQ receptor to an opioid receptor using four point mutations. J Biol Chem. 1996;271(50):32016–20.
Meng F, Ueda Y, Hoversten MT, Taylor LP, Reinscheid RK, Monsma FJ, et al. Creating a functional opioid alkaloid binding site in the orphanin FQ receptor through site-directed mutagenesis. Mol Pharmacol. 1998;53(4):772–7.
Lipiński PFJ, Kosson P, Matalińska J, Roszkowski P, Czarnocki Z, Jarończyk M, et al. Fentanyl family at the Mu-opioid receptor: Uniform Assessment of Binding and Computational Analysis. Molecules. 2019;24(4).
Koehl A, Hu H, Maeda S, Zhang Y, Qu Q, Paggi JM, et al. Structure of the μ-opioid receptor-G(i) protein complex. Nature. 2018;558(7711):547–52.
Zaveri NT, Marquez PV, Meyer ME, Polgar WE, Hamid A, Lutfy K. A novel and selective nociceptin receptor (NOP) agonist (1-(1-((cis)-4-isopropylcyclohexyl) piperidin-4-yl)-1H-indol-2-yl)methanol (AT-312) decreases acquisition of ethanol-induced conditioned place preference in mice. Alcohol Clin Exp Res. 2018;42:461–71.
Zaveri NT, Marquez PV, Meyer ME, Hamid A, Lutfy K. The nociceptin Receptor (NOP) agonist AT-312 blocks acquisition of morphine- and cocaine-induced conditioned place preference in mice. Front Psychiatry. 2018;9:638.
Thompson AA, Liu W, Chun E, Katritch V, Wu H, Vardy E, et al. Structure of the nociceptin/orphanin FQ receptor in complex with a peptide mimetic. Nature. 2012;485(7398):395–9.
Miller RL, Thompson AA, Trapella C, Guerrini R, Malfacini D, Patel N, et al. The importance of ligand-receptor conformational pairs in stabilization: spotlight on the N/OFQ G protein-coupled receptor. Structure. 2015;23(12):2291–9.
Acknowledgements
This work was supported by grants R01DA027811 (N.T.Z.), R43NS070664 (N.T.Z.), R44DA042465 (N.T.Z) and NIAAA Contracts HHSN275201300005C and HHSN275201500005C from the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
M.E.M and A.D. contributed equally to this work. M.E.M, A.D., and D.Y. conducted the design, chemical synthesis, and SAR analysis of the NOP ligands. A.D. conducted the molecular docking experiments. N.T.Z conducted the design and the SAR and data analysis, and supervised the study.
Corresponding author
Additional information
Guest Editors: Diane Burgess, Marilyn Morris and Meena Subramanyam
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 3418 kb)
Rights and permissions
About this article
Cite this article
Meyer, M.E., Doshi, A., Yasuda, D. et al. Structure-Based SAR in the Design of Selective or Bifunctional Nociceptin (NOP) Receptor Agonists. AAPS J 23, 68 (2021). https://doi.org/10.1208/s12248-021-00589-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-021-00589-7