Abstract
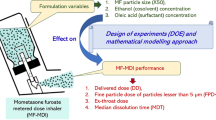
Metered dose inhalers (MDIs) are complex drug-device combination products widely used to treat pulmonary disorders. The efficacy, driven by aerosol performance of the products, depends on a multitude of factors including, but not limited to, the physicochemical properties of drug and nature and amount of excipient(s). Under the quality by design (QbD) paradigm, systematic investigations are necessary to understand how changes in critical quality attributes (CQAs) of formulation, device, and manufacturing process influence key product performance parameters, such as delivered dose (DD) and fine particle dose (FPD). The purpose of this work is to provide a better understanding of the effects of different levels of excipients and drug particle size distribution on the aerosol performance of MDI products, while using two fundamentally different MDI products as relevant model systems, Proventil® HFA (albuterol sulfate suspension) and Qvar® (beclomethasone dipropionate solution). These MDI products, as model systems, provided mid-points around which a design of experiments (DOE), consisting of 22 suspension and 9 solution MDI formulations, were defined and manufactured. The DOE included formulations factors with varying ethanol (2 to 20% w/w and 7 to 9% w/w for the suspension and solution, respectively) and oleic acid concentrations (0.005 to 0.25% w/w and 0 to 2% w/w for the suspension and solution, respectively) and drug volumetric median particle size distribution (PSD D50, 1.4 to 2.5 μm for the suspension). The MDI formulations were analyzed using compendial methods to elucidate the effect of these formulation variables (ethanol, oleic acid, and PSD D50) on DD and FPD. The outcomes of this study allowed defining design spaces for the formulation factors, such that DD and FPD would remain within specific pre-defined requirements. The systematic approach utilized in this work can contribute as a QbD tool to evaluate the extent to which the formulation factors govern the aerosol performance of MDI drug products, helping to design MDI formulations with desired product performance parameters.






Similar content being viewed by others
References
Myrdal PB, Sheth P, Stein SW. Advances in metered dose inhaler technology: formulation development. AAPS PharmSciTech. 2014;15(2):434–55.
Ivey JW, Vehring R, Finlay WH. Understanding pressurized metered dose inhaler performance. Expert Opinion Drug Deliv. 2015;12(6):901–16.
Stein SW, Sheth P, Hodson PD, Myrdal PB. Advances in metered dose inhaler technology: hardware development. AAPS PharmSciTech. 2014;15(2):326–38.
Borgström L. The pharmacokinetics of inhaled hydrofluoroalkane formulations. J Allergy Clin Immunol. 1999;104(6):s246–s9.
US Pharmacopeia. General chapters: <601> inhalation and nasal drug products: aerosols, sprays, and powders—performance quality tests. Official from May 1, 2015; USP 38-NF 35:388–414.
Food and Drug Administration - Office of Generic Drugs. Albuterol sulfate inhalation aerosol metered. Draft Guidance. 2016.
Copley M. Improving the realism and relevance of mouth-throat models for inhaled product testing. www.ondrugdelivery.com. 2015; 32–7.
Shur J, Saluja B, Lee S, Tibbatts J, Price R. Effect of device design and formulation on the in vitro comparability for multi-unit dose dry powder inhalers. AAPS J. 2015;17(5):1105–16.
US Department of Health and Human Services - Food and Drug Administration. Metered dose inhaler (MDI) and dry powder inhaler (DPI) drug products—chemistry, manufacturing, and controls documentation. Guidance for Industry—Draft. 1998; 65.
Smyth HDC. The influence of formulation variables on the performance of alternative propellant-driven metered dose inhalers. Adv Drug Deliv Rev. 2003;55(7):807–28.
Lionberger RA, Lee SL, Lee L, Raw A, Lawrence XY. Quality by design: concepts for ANDAs. AAPS J. 2008;10(2):268–76.
Smith IJ, Bell J, Bowman N, Everard M, Stein S, Weers JG. Inhaler devices: what remains to be done? J Aerosol Med Pulm Drug Deliv. 2010;23(S2):S-25–37.
Conti DS, Grashik J, Yang L, Wu L, da Rocha SRP. Solvation in hydrofluoroalkanes: how can ethanol help? J Pharm Pharmacol. 2011;64(9):1236–44.
Zhu B, Traini D, Young P. Aerosol particle generation from solution-based pressurized metered dose inhalers: a technical overview of parameters that influence respiratory deposition. Pharm Dev Technol 2014(0):1–14.
Stein S, Myrdal P. The relative influence of atomization and evaporation on metered dose inhaler drug delivery efficiency. Aerosol Sci Technol. 2006;40(5):335–47.
Zhu B, Traini D, Chan H-K, Young PM. The effect of ethanol on the formation and physico-chemical properties of particles generated from budesonide solution-based pressurized metered-dose inhalers. Drug Dev Ind Pharm. 2013;39(11):1625–37.
Zhu B, Traini D, Lewis DA, Young P. The solid-state and morphological characteristics of particles generated from solution-based metered dose inhalers: influence of ethanol concentration and intrinsic drug properties. Colloids Surf A Physicochem Eng Asp. 2014;443:345–55.
Saleem IY, Smyth HD. Tuning aerosol particle size distribution of metered dose inhalers using cosolvents and surfactants. BioMed Res Int. 2013;2013:1–7.
Stein SW, Myrdal PB. A theoretical and experimental analysis of formulation and device parameters affecting solution MDI size distributions. J Pharm Sci. 2004;93(8):2158–75.
Gupta A, Stein SW, Myrdal PB. Balancing ethanol cosolvent concentration with product performance in 134a-based pressurized metered dose inhalers. J Aerosol Med. 2003;16(2):167–74.
Williams RO III, Liu J. Formulation of a protein with propellant HFA 134a for aerosol delivery. Eur J Pharm Sci. 1999;7(2):137–44.
Pu Y, Kline LC, Khawaja N, Van Liew M, Berry J. Comparison of optical particle sizing and cascade impaction for measuring the particle size of a suspension metered dose inhaler. Drug Dev Ind Pharm. 2015;41(5):737–43.
Sheth P, Stein SW, Myrdal PB. Factors influencing aerodynamic particle size distribution of suspension pressurized metered dose inhalers. AAPS PharmSciTech. 2014; 1–10.
Vervaet C, Byron PR. Drug-surfactant-propellant interactions in HFA-formulations. Int J Pharm. 1999;186(1):13–30.
Williams RO III, Liu J. Influence of formulation additives on the vapor pressure of hydrofluoroalkane propellants. Int J Pharm. 1998;166(1):99–103.
Zhu B, Xu N, Traini D, Lewis D, Young PM. The formation of aerosol particles from solution-based pressurized metered dose inhalers and implications of incomplete droplet drying: theoretical and experimental comparison. Aerosol Sci Technol. 2015; doi:10.1080/02786826.2015.1096897.
Mitchell J, Copley M, Sizer Y, Russell T, Solomon D. Adapting the Abbreviated Impactor Measurement (AIM) concept to make appropriate inhaler aerosol measurements to compare with clinical data: a scoping study with the “Alberta” Idealized Throat (AIT) inlet. J Aerosol Med Pulm Drug Deliv. 2012;25(4):188–97.
Guo C, Gillespie SR, Kauffman J, Doub WH. Comparison of delivery characteristics from a combination metered-dose inhaler using the Andersen cascade impactor and the next generation pharmaceutical impactor. J Pharm Sci. 2008;97(8):3321–34.
ACKNOWLEDGMENTS
Funding for this work was made possible, in part, by the Food and Drug Administration through grant 1U01FD004943-01. Views expressed in this publication do not necessarily reflect the official policies of the Department of Health and Human Services, nor does any mention of trade names, commercial practices, or organization imply endorsement by the US Government.
Author information
Authors and Affiliations
Corresponding author
Additional information
The opinions expressed in this paper by the authors do not necessarily reflect the views or policies of the Food and Drug Administration (FDA).
Rights and permissions
About this article
Cite this article
Sheth, P., Sandell, D., Conti, D.S. et al. Influence of Formulation Factors on the Aerosol Performance of Suspension and Solution Metered Dose Inhalers: A Systematic Approach. AAPS J 19, 1396–1410 (2017). https://doi.org/10.1208/s12248-017-0095-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-017-0095-3