Abstract
Although using spray-dried dispersions (SDDs) to improve the bioavailability of poorly water-soluble compounds has become a common practice in supporting the early phases of clinical studies, their performance evaluation, whether in solid dosage forms or alone, still presents significant challenges. A microcentrifuge dissolution method has been reported to quickly assess the dissolution performance of SDDs. While the microcentrifuge dissolution method has been used in the SDD community, there is still a need to understand the mechanisms about the molecular species present in supernatant after centrifugation, the molecular nature of active pharmaceutical ingredients (APIs), as well as the impact of experimental conditions. In this paper, we aim to assess the effect of API and polymer properties on the dissolution behavior of SDDs along with centrifuging parameters, and for this, two poorly water-soluble compounds (indomethacin and ketoconazole) and two commonly used polymers in the pharmaceutical industry (PVP and HPMC-AS) were chosen to prepare SDDs. A typical microcentrifuge dissolution procedure as reported in the publication (Curatolo et al., Pharm Res 26:1419–1431, 2009) was followed. In addition, after separation of the supernatant from precipitation, some of the samples were filtered through filters of various sizes to investigate the particulate nature (particle size) of the supernatant. Furthermore, the centrifuge speed was varied to study sedimentation of API, SDD, or polymer particles. The results indicated that for the SDDs of four drug-polymer pairs, microcentrifuge dissolution exhibited varied behaviors, depending on the polymer and the drug used. The SDDs of indomethacin with either PVP or HPMC-AS showed a reproducible dissolution with minimum variability even after filtration and subjecting to varied centrifugation speed, suggesting that the supernatant behaved solution-like. However, ketoconazole-PVP and ketoconazole-HPMC-AS SDDs displayed a significant variation in concentration as the speed of centrifugation and the pore sizes of filters were altered, indicating that their supernatant was heterogeneous with the presence of particulates. In conclusion, microcentrifuge dissolution method was more suitable for indomethacin-PVP and indomethacin-HPMC-AS systems compared with ketoconazole-PVP and ketoconazole-HPMC-AS. Therefore, the use of microcentrifuge dissolution method depends on both compounds and polymers selected, which should be examined case by case.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
As poorly water-soluble compounds account for a substantial portion of portfolios from discovery, delivery of these compounds becomes critical since solubility is often the key barrier for achieving bioavailability in many cases (1–4). Among the technologies available for improving bioavailability-particle size reduction, solubilization, lipid drug delivery system, melt extrusion, and spray-dried dispersions (SDDs), SDD is the most commonly used, due to its unique attributes including the easiness of preparation, universality (applicable to many compounds), and commercially available equipment. During the preparation of SDDs, crystalline drugs are typically converted to the amorphous form or the supercool liquid state in order to increase their apparent solubility (5–9). As molecules in the amorphous state are less stable compared with crystalline material, polymers are incorporated in SDDs to improve their stability. In addition, some polymers such as hydroxypropyl methylcellulose acetate succinate (HPMC-AS) can interact with drug molecules and maintain supersaturation after dissolution, which further enhances the bioavailability of drugs (10,11). However, relative to the dissolution test of conventional dosage forms, the evaluation of SDDs, in particular the supersaturation state of drugs, poses significant challenges. In order to assess the supersaturation state, a microcentrifuge method (MCD) was developed, a practical method to evaluate the performance of SDDs. To perform MCD test, SDD samples are typically suspended in dissolution media in centrifuge tubes, followed by vortexing to ensure the content uniformity. After applying centrifugal force (centrifuging for certain time at certain speed), particles are separated from supernatant by sedimentation. With the assumption that all contents in supernatant are potentially bioavailable, they are analyzed using HPLC after dissolving in organics. Compared with the conventional dissolution (CD) that solution samples are taken by filtering through a 0.45-μm filter followed by HPLC analysis in which the paddle speed is one of important parameters to consider, MCD relies on sedimentation to separate supernatant from precipitates. Therefore, for MCD, centrifuging speed is the most critical factor in influencing active ingredient concentration in supernatant. More importantly, it is crucial to know the speed which yields an equivalent dissolution as CD if comparison is deemed to be necessary. In addition, mechanistically, MCD method needs to be further investigated, especially in terms of the impact of centrifugation speed as well as the effect of the particulate nature of supernatants on dissolution profiles.
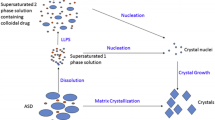
For the SDDs of drug-polymer pairs, particularly those prepared with HPMC-AS, it has been hypothesized that after dissolution, drugs and polymers exist as many microstates named species (11). According to species postulation, during dissolution, drugs along with polymers are only partially dissolved to form molecules, whereas the rest is dispersed as nano/colloidal particles, or even macroparticles (significantly greater than 1 μm). Presumably, the interactions between drugs and polymers are responsible for maintaining supersaturation, producing higher apparent solubility. However, it is still unclear that how nano/ colloidal particles contribute to solubility and bioavailability improvement. During MCD, the API concentration in supernatant after centrifugal separation is measured at each time point, where large particles are typically forced to settle while molecules or small particles, not affected by the centrifugal force, remain in the supernatant. In this process, it appears that the speed and the time used for centrifuging are critical since they control the size and the number of small particles retained in the supernatant, in which 13,000×g was typically used for HPMC-AS SDDs (11).
Additionally, it is desirable to compare MCD with CD (12) which can be used to facilitate method development in late stage product development. Therefore, it is important to understand the impact of centrifuging parameters on the measured concentration of APIs in the supernatant in addition to the applicability of this method to SDDs with a wide range of physical properties. To investigate the impact of API and polymer properties on the MCD results, we identified two pharmaceutical polymers, polyvinylpyrrolidone (PVP) and hydroxypropyl methylcellulose acetate succinate (HPMC-AS), as well as two drugs, indomethacin and ketoconazole (IMC and Keto) to prepare SDDs. Additionally, to understand the impact of centrifugation parameters, the centrifuge speed was varied from 6000 to 16,000×g. Furthermore, by comparing the drug concentration in supernatant before and after filtration when various pore sizes of filters were used, the impact of filtration along with the pore size of filters can be assessed. Moreover, kinetic solubility was performed to compare with MCD results, further clarifying the effect of particulate nature of supernatant on dissolution profile. In terms of dissolution media, pH 6.8 phosphate buffer solution and simulated gastric fluid were used. Finally, based on these results, a mechanistic model is suggested to explain the dissolution behaviors of SDDs, particularly for SDDs generating particulates during dissolution.
MATERIAL AND METHODS
Chemicals
Indomethacin and ketoconazole were purchased from Sigma-Aldrich Chemical Inc. (Missouri, USA) and Spectrum Chemicals Inc. (California, USA), respectively. Their physical chemical properties are listed in Table I. Polyvinylpyrrolidone (PVP) was a gift from Ashland Chemicals Inc. (Kentucky, USA), and hydroxypropyl methylcellulose acetate succinate (HPMC-AS) was obtained from Shin-Etsu Chemicals Inc. (Japan). According to the specifications from Shin-Etsu, HPMC-AS contains 23.4% methoxyl, 7.2% hydroxypropyl, 9.4% acetyl, and 11.0% succinoyl (Mw = 8.0 × 104, Mn = 4.4 × 104). Solvents used for spray drying include acetone and methanol. Both were from Sigma-Aldrich Chemicals Inc. All of the SDD formulations were prepared internally using a 1:3 (w/w) API to polymer ratio. Methanol was used in sample dilution, and acetonitrile as well as ammonium acetate were used for preparing for HPLC mobile phase. All of them were from Sigma-Aldrich Chemicals Inc.
SDD Sample Preparation
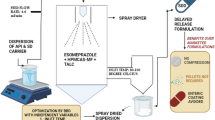
In general, SDD samples were prepared by dissolving drug-polymer pairs in their corresponding solvent/solvents followed by spray drying. As displayed in Table II, four SDDs prepared during this study all consist of 25% API (w/w). For preparing IMC-HPMC-AS dispersions, 3.3% (w/v) of IMC-HPMC-AS solution in acetone was first made by dissolving 40 g of a drug-polymer mixture in acetone with a total volume of 1200 mL followed by spray drying. Similarly, 3.3% of IMC-PVP solution in methanol was prepared for generating the IMC-PVP SDD material. Since ketoconazole and HPMC-AS cannot be dissolved in either acetone or methanol, a mixed solution of acetone and methanol (50:50, v/v) was used. Same as preparing for the IMC-PVP solution, methanol was used for preparing the Keto-PVP spray drying solution. After preparing these drug/polymer solutions, they were then dried using a Buchi model 290 laboratory spray drier (Buchi Corporation, New Castle, DE, USA). Spray drying conditions include the following: aspiration (73%), pump rate (8%), nozzle cleaning, as well as inlet temperature (60–63°C) for acetone as solvent and (70–73°C) for methanol as solvent. For all preparations, the flow rate of inert gas, nitrogen, was kept 40 mL/min. Prior to any measurements, all SDD samples were vacuum-dried overnight at ambient temperature to ensure that residual solvent was completely removed.
Dissolution
For all dissolution experiments, 50 mM potassium phosphate buffer of pH 6.8, purchased from CHATA Biosystems (Loveland, Colorado, USA 80538), was used as a dissolution medium. The biorelevant medium, FASSIF, was prepared by dissolving 112 mg of FASSIF powder (Biorelevant Co., Croydon, Surrey, UK), 30.9 mg of sodium chloride (Cat No. 12314, Alfa Aesar, MA, USA), and 50 mL of potassium phosphate buffer, pH 6.8.
Microcentrifuge Dissolution
A 10–11 mg sample of SDD was first weighed into a 1.5-mL microcentrifuge tube (Eppendorf) followed by addition of 1.5 mL of the dissolution medium. After the tube was capped, a timer was immediately initiated. Then, the tube was vortexed at the highest intensity for 60 s (VWR Vortex - Genie 2) before placing it in a block heater (Eppendorf Thermomixer 5436) at 37°C for 16 min or specified time per study. At the end of settling time period, the tube was removed from the block heater and put in a microcentrifuge (Eppendorf Microcentrifuge 5415D) for centrifuging. Centrifuging speed was set at 13,000×g or specified speed per study for 1 min, and the tube was left in the centrifuge until reaching the sampling time point (20 min). Then, the tube was removed, and 25 μL of supernatant was withdrawn and transferred into a HPLC vial followed by addition of 1 mL of the diluent (methanol/50 mM potassium phosphate buffer, pH 6.8 50/50) and vortexing before HPLC injection. The same procedure was applied to other time points such as 60, 120, and 180 min. A typical scheme for the MCD process is shown in the Table III.
For MCD filtration study, a similar procedure was followed with an additional filtration step using various sizes of filters. Again, 25 μL of the filtered supernatant was withdrawn from the tube and injected in HPLC for analysis after dilution. For all analysis, HPLC system used was Agilent HP1100 system equipped with UV detector and Phenomenex Luna C18 column. The mobile phase consists of acetonitrile and 20 mM ammonium acetate. The detection wavelength was 318 nm for indomethacin and 223 nm for ketoconazole.
Kinetic Dissolution
The kinetic dissolution study was conducted by weighing 100 mg of SDDs into a 20-mL scintillation vial, followed by addition of 15 mL of dissolution medium (phosphate buffer, pH 6.8). After continuously shaking the vials at ambient temperature for the specified time using a mechanical shaker, 1.5 mL solution was withdrawn for analysis at the following time points: 20, 60, 120, and 180 min. Before HPLC analysis, all samples were filtered through an Acrodisc PVDF 0.45-μm filter.
RESULTS
Impact of Filtration
Contrary to the conventional dissolution (12) that majority of particles are excluded by filtration, during MCD measurement, the amount of particles in supernatant depends on the centrifugal speed used. According to speciation hypothesis, after sedimentation, the supernatant may consist of free drug molecules, drug particles, polymeric molecules, polymer particles, and drug-polymer particles of various sizes. In fact, the particulate nature of the supernatant, particulate species are retained in the supernatant, is decided by factors, including the physical properties of both APIs and polymers, as well as the processing parameters for centrifuging. Because the MCD is different from the CD where samples for analysis are typically filtered through a 0.45-μm filter, it is interesting to investigate the effect of filtration on the MCD results. Particularly, the conventional dissolution is a well-controlled method, having a well-established regulatory status as described in USP <1092> Dissolution Procedure: Development and Validation. Since the MCD test is only a discovery tool with limited method development and no validation, a correlation between the two could serve as an indicator of validity for the MCD method. To investigate the effect caused by filtration, the supernatants of all four SDDs (IMC-PVP, IMC-HPMC-AS, Keto-PVP, and Keto-HPMC-AS) are filtered. As indicated in Table IV, for the SDDs of IMC-PVP and IMC-HPMC-AS, the API concentration before and after filtration (0.45 μm) is very similar, indicating that after centrifuging at 13,000×g, all particles larger than 0.45 μm are removed. These results should be considered as comparable to those obtained from the CD (12). One exception was noted for the SDD made of ketoconazole with either PVP or HPMC-AS that a significant discrepancy between the filtered and non-filtered samples was observed. As displayed in Table IV for Keto-HPMC-AS, after filtration, the concentration of ketoconazole was reduced to less than 10% in comparison with the non-filtered sample, implying existence of large particles in the supernatant. This raised a question about the size of particles in the supernatant. To investigate this phenomenon, we selected the Keto-HPMC-AS SDD to be further studied using filters with pore sizes ranged from 0.2 to 1.2 μm. Table V shows the ketoconazole concentration prior and post filtration. According to Table V, the API concentration after filtration increases with filter size, suggesting that the supernatant contains various sizes of particles. To the extreme, when 0.2-μm Nylon filter was used, the drug concentration became extremely low. However, when 1.2-μm filter was used, the API concentration before and after filtration appears to be comparable, revealing the fact that particles above this size were centrifuged down. The above results strongly indicate that for the SDDs of either IMC-PVP or IMC-HPMC-AS, the MCD and the CD should yield compatible dissolution results, whereas for the SDDs of both Keto-PVP and Keto-HPMC-AS, the API concentration in the supernatant is affected by both the properties of polymers used and the physical properties of API, in particular the physical properties of drugs. In the case of Keto-HPMC-AS, it seems that dissolution was significantly impacted by HPMC-AS. In this case, it appears that both ketoconazole and HPMC-AS critically influence the MCD dissolution behavior of SDDs at a speed of 13,000×g.
Effect of Centrifuge Speed
For SDD suspensions with various sizes of particles, particle sedimentation during centrifugation mainly on the power of centrifuge brings down particles against the buoyant force. This is also true for the MCD, where 13,000×g is used since it is assumed that at this speed, most of the particles, if not all, are precipitated. Therefore, the supernatant collected can be regarded as closed to the solution similar to that obtained from the CD. This might be true for some SDDs. However, the particulate nature of SDDs, as demonstrated in the last section, is affected by the properties of both APIs and polymers. To test this hypothesis, varied centrifuge speed was used, and the results are reported in this section. As shown in Table VI, for the SDDs of two drug-polymer pairs, IMC-PVP and IMC-HPMC-AS, lowering the power of centrifugation from 13,000 to 5000×g has a limited impact on the API concentration in their corresponding supernatant, essentially suggesting that even at 5000×g, most of the precipitable particles in the supernatant of these SDDs are centrifuged down. In other words, this implies two scenarios: (1) SDD dispersions consist of molecules and nanoparticles that they are not settled even at 13,000×g force and (2) SDD dispersions have larger particles that are precipitated at 5000×g force. Alternatively, this means that there were no mid-size particles present. On the contrary, the SDDs of Keto-PVP and Keto-HPMC-AS exhibited a very different behavior. As seen in Table VI, for Keto-HPMC-AS, the ketoconazole concentration in the supernatant is substantially higher when 5000×g was used, comparing to 13,000×g. This is expected since lower centrifuge speed could leave more particles in the supernatant. Additionally, the API concentration for the Keto-PVP SDD is not only comparable at both speeds (5000 and 13,000×g) but also very low relative to other three SDDs. To explore the influence of centrifuge power on the API concentration during MCD, the ketoconazole concentration in the supernatant was examined for the SDDs of Keto-HPMC-AS after centrifugation using different speeds. In Table VII, the measured API concentration increased when the centrifuge power was lowered although the API concentration is comparable at the centrifuge power of 13,000 and 15,400×g. So, from centrifuge power point of view, 13,000×g is an optimum speed given that the upper limit of operating range for most of microcentrifuge equipments is 16,000×g. Nonetheless, as demonstrated in the section, even using 13,000×g cannot ensure a complete removal of all particles for the Keto-HPMC-AS SDD. In conclusion, although 13,000×g is the optimum centrifuge speed which efficiently works for some of SDDs of drug-polymer pairs, for the Keto-HPMC-AS SDDs, it still cannot ensure the removal of some submicron particles. This reflects the heterogeneity of particulate nature of SDD suspensions after dissolution, proving that MCD as a dissolution method should be used with caution.
Impact of Dissolution Medium
Although the MCD dissolution of SDDs of indomethacin and ketoconazole with PVP and HPMC-AS in the phosphate buffer revealed that both drugs and polymers affect their dissolution behavior, it is desired to investigate their behavior in a biorelevant medium. To answer this question, MCD dissolution was performed in a biorelevant medium, FASSIF. In Fig. 1a, b, MCD dissolution profiles for Keto-PVP and Keto-HPMC-AS are shown, where the profiles in FASSIF are displayed over the profiles in the phosphate buffer.
For both SDDs, their dissolution in FASSIF is much higher in relation to that in the phosphate buffer. This is true for both SDDs; for both cases, the API concentration of Keto-HPMC-AS is substantially higher relative to that of Keto-PVP. This suggests that HPMC-AS plays a better role to maintain supersaturation compared with PVP. Additionally, based on the results from this paper, it also shows that the dissolution of Keto-HPMC-AS generates many particles around 1 μm, which can be filtered by 0.45 μm but cannot be removed by centrifugation. Given that MCD is very sensitive to the presence of particulates, in order to relate MCD with conventional dissolution method (12), it is necessary to compare MCD with kinetic solubility.
Kinetic Dissolution of SDDs
In addition to the results reported above, it is also beneficial to compare the MCD with kinetic solubility in the same buffer since it can reveal the stabilization of API molecules by polymers up to 3 h, which is a typical time of nucleation without any inhibition. The kinetic dissolution conducted in this study was extended to 3 h in order to observe supersaturation profiles. As shown in Fig. 2a, b, the kinetic dissolution profiles of IMC-PVP and IMC-HPMC-AS display a similar trend as those from the MCD, remaining constant with time, although for the IMC-PVP SDD, the IMC concentration in the supernatant is lower relative to that from kinetic dissolution. On the other hand, for the IMC-HPMC-AS SDD, the API concentration value is comparable even though the IMC concentration from the MCD is a little higher. Interestingly in both cases, the API concentration stays the same from 20 to 180 min, indicating that for the IMC SDDs with both polymers, drug molecules are not strongly held by polymers where IMC can be quickly dissolved or solvated in aqueous media once SDDs contact the medium. Alternately, the strong interaction between drugs and polymer molecules could lead to longer supersaturation period of drugs in the dissolution medium. However, for the SDDs of ketoconazole, whether with PVP or HPMC-AS, both the kinetic dissolution and the MCD profiles exhibit a different trend as shown in Fig. 3a, b. First, in both cases, the ketoconazole concentration in the supernatants measured from the MCD is much higher, and in addition, it decreases with time for both SDDs, indicating drug molecules gradually precipitate out from their supersaturation state. In the case of the Keto-HPMC-AS SDD, there is a significant concentration drop observed after 20 min, in which the API concentration eventually coincides with that of solubility measurement. Relatively for the Keto-PVP SDD, the API concentration drop in the supernatant is not much less as compared to that of Keto-HPMC-AS, due to a lower solubility in general. Additionally, in this case, the API concentration from the solubility measurement not only is lower but also decreases with time, suggesting ketoconazole precipitates from supersaturation after certain time. Furthermore, the results from both Keto-PVP and Keto-HPMC-AS SDDs demonstrate that besides API, polymers used in SDDs can greatly affect their MCD dissolution behavior. The less hydrophilic property of HPMC-AS is also for the higher solubility of keto-HPMC-AS dispersion in the kinetic study. Overall, by comparing the MCD with kinetic dissolution, we can conclude that the physical properties of both APIs and polymers in SDDs can impact their dissolution behavior.
DISCUSSION
Comparing with the conventional dissolution test of crystalline drugs, the MCD dissolution is fundamentally different. When performing dissolution test on tablets of crystalline API, essentially, it is the API dissolving process being monitored, with a concentration-time plot revealing the rate of API dissolving process. In contrast, APIs in SDDs are in molecular state, which changes their dissolving process into a solvation process with no breaking of lattice structures. In the presence of polymers, wetting of SDD particles, in addition to nucleation inhibition, can be significantly improved. Thus, the dissolution of SDD particles involves particle wetting, swelling of SDD particles, essentially swelling of polymers, solvation of APIs, as well as solvation and dissolving of polymers. Depending on the hydrophilic nature of polymers used and their solution structures as well as the physical chemical properties of APIs, the particulate nature of SDDs, including particle size, size distribution, and nature of these particles, after dissolution in the media, is very different. For example, relative to HPMC-AS, hydrophilic polymers like PVP tend to dissolve quickly, often leaving drug molecules unprotected. In addition, if APIs like IMC can be solvated rapidly, this effect is less since APIs are swiftly turned into solvated molecular state. In terms of polymer solution structures, due to its strong interaction with water, PVP has a typical random coil structures in aqueous solution, which probably does not allow the polymer to interact with APIs favorably. However, for SDDs of HPMC-AS, particularly with drugs of poor solubility and wettability, the situation is very different; given that HPMC-AS is less hydrophilic than PVP, its solvation process takes longer as well. In this case, since SDD particles are slowly solvated, relatively, particles of SDDs remain in solution much longer before polymers are dissolved away. In addition, also due to this slow solvation process, the initial SDD particles can be broken down to form various sizes of drug-polymer particles. Moreover, HPMC-AS has a very different solution structure compared with PVP. Due to low surface tension nature of HPMC-AS solution, it is likely that HPMC-AS forms aggregates in solution.
Although MCD has its advantage of being simple and eliminating many steps such as filtration, operation wise, many precautious steps need to be considered for successfully performing MCD. Particularly, since SDD particles tend to settle which will limit the exposure of these particles to the dissolution media, it is very critical to keep them from firmly settling. To generate reproducible data, sufficient vortexing is essential for executing MCD since vortexing is the step to break solid precipitate after centrifugation. The amount of vortexing must be understood, and this is a method parameter which should be developed and understood. The time for vortexing, settling in a 37°C heating block as well as centrifuging has to be consistent from sample to sample. The solution in centrifuge tube is rather viscous. Reverse pipetting is recommended for pipetting 25 μL of supernatant precisely and accurately. In the publication (11), the whole process from adding medium to centrifuge tube to pipetting 25 μL supernatant for dilution was conducted in a temperature-controlled box at 37°C. We kept the centrifuge tube in a 37°C conditions only in the step of settling as this is the longest and heat-releasing step. Both vortexing and centrifuging are heat-generating steps and only last for 1 min.
CONCLUSIONS
In this study, it is demonstrated that MCD is practically a useful method for evaluating SDDs, but it has limited applicability as shown by the results from this paper. Depending on the properties of APIs and polymers used, the concentration from filtered supernatant can be significantly less relative to the unfiltered. In addition, the centrifuging speed can considerably affect the API concentration in supernatant. Furthermore, kinetic solubility study strongly supports that both API properties and polymer nature can influence the behavior of the MCD dissolution of SDDs. Based on our experience in practice, without polymer, the API concentration in supernatant is seldom detectable—completely settled after centrifugation. Overall, the results from this study show that the particulate nature of supernatant in MCD is strongly affected by both APIs and polymers, which is controlled by the wettability and dissolution behavior of both API and polymer, as well as their solubility and solution structure. This in turn impacts their results from MCD. Therefore, it is important to examine the particulate nature of supernatants in MCD and the propensity of forming particulates when SDDs are in contact with aqueous media before adopting MCD as a routine evaluation method. Previous studies had been reported on the parameters affecting supersaturation such as temperature (13,14). Reports have shown that the supersaturation profiles are affected by the changes of drug load in SDD (11,15,16,17). The studies we presented here show that supersaturation profiles are affected by the revolutions per minute speed of centrifuge, the centrifuge time, the filtration of supernatant. Using high speed of microcentrifuge has the advantage of avoiding the step of supernatant filtration and consequently can perform sampling from the same vial for all time points. It appears that the filtration step is needed even in the case of suggested 13,000×g centrifuge power. If step of filtration is inevitable, a conventional centrifuge at the level of 3000 to 5000×g may be the appropriate one to use. The MCD method parameters can have significant effect on the result. The MCD procedure should only be used as a discovery tool if these parameters are not fully understood and investigated as part of method development.
REFERENCES
Lipinski CA, Lombardo F, Dominy BW, Feeny PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2012;64 Suppl 1:4–17.
Serrajuddin ATM. Solid dispersions of poorly water-soluble drugs: early promises, subsequent problems and recent breakthroughs. J Pharm Sci. 1999;88:1058–66.
Brouwers J, Brewster ME, Augustijns P. Supersaturating drug delivery systems: answer to solubility-limited oral bioavailability. J Pharm Sci. 2009;98:2549–72.
Brown CK, Chokshi HP, Nickerson B, Reed RA, Rohrs BR, Shah PA, et al. Acceptable analytical practices for dissolution testing of poorly soluble compounds. Pharm Technol. 2004:56–65.
Newman A, Knipp G, Zografi G. Assessing the performance of amorphous solid dispersions. J Pharm Sci. 2012;101(4):1355–77.
Yu L. Amorphous pharmaceutical solids: preparation, characterization and stabilization. Adv Drug Deliv Rev. 2001;48:27–42.
Friesen DT, Shanker R, Crew M, Smithey DT, Curatolo WJ, Nightingale JAS. Hydroxypropyl methylcellulose acetate succinate-based spray-dried dispersions: an overview. Mol Pharm. 2008;5(6):1003–19.
Paudel A, Worku ZA, Meeus J, Guns S, Mooter GVD. Manufacturing of solid dispersions of poorly water soluble drugs by spray drying: formulation and process considerations. Int J Pharm. 2012;453:253–84.
Li J, Zhao J, Tao L, Wang J, Waknis V, Pan D, et al. The effect of polymeric excipients on the physical properties and performance of amorphous dispersions: part I, free volume and glass transition. Pharm Res. 2015;32:500–15.
Ueda K, Higashi K, Kataoka M, Yamashita S, Yamamoto K, Moribe K. Inhibition mechanism of hydroxypropyl methylcellulose acetate succinate on drug crystallization in gastrointestinal fluid and drug permeability from a supersaturation solution. Eur J Pharm Sci. 2014;62:293–300.
Curatolo W, Nightingale JA, Herbig SM. Utility of hydroxypropylmethylcellulose acetate succinate (HPMCAS) for initiation and maintenance of drug supersaturation in the GI milieu. Pharm Res. 2009;26:1419–31.
Chapter 711: Dissolution. United States Pharmacopeia 39 (USP 39): National Formulary 34 (NF 34). 2015.
Alonzo DE, Zhang GGZ, Zhou D, Gao Y, Taylor LS. Understanding the behavior of amorphous pharmaceutical systems during dissolution. Pharm Res. 2010;27(4):608–18.
Sun DD, Lee PI. Evolution of supersaturation of amorphous pharmaceuticals: the effect of rate of supersaturation generation. Mol Pharm. 2013;10:4330–46.
Langham ZA, Booth J, Hughes LP, Reynolds GK, Wren SAC. Mechanical insights into the dissolution of spray-dried amorphous solid dispersions. J Pharm Sci. 2012;101(8):2798–810.
Qian F, Wang J, Hartley R, Tao J, Haddadin R, Mathias N, et al. Solution behavior of PVP-VA and HPMC-AS-based amorphous solid dispersions and their bioavailability implications. Pharm Res. 2012;29:2766–76.
Eerdenbrugh BV, Alonzo DE, Taylor LS. Influence of particle size on the ultraviolet spectrum of particulate-containing solutions: Implications for in-situ concentration monitoring using UV/Vis fiber-optic probes. Pharm Res. 2011;28:1643–52.
ACKNOWLEDGMENTS
The authors would like to thank Interfacial Science Research Team of DPST at BMS for providing SDD samples. The author BW would like to acknowledge Pedro Smith of DPST at BMS for generously letting him use the work space and equipments during the study and Dr. Xujin Lu and Lili Lo of ABD at BMS for the valuable discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
All financial support for the studies reported herein was provided by Bristol-Myers Squibb. The authors have no financial involvement with any other organization or entity with a financial interest in or financial conflict with the subject matter or material discussed in the manuscript. This includes consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. No writing assistance was utilized in the production of this manuscript.
Additional information
Guest Editors: Otilia M. Koo, Panayiotis P. Constantinides, Lavinia M. Lewis, and Joseph Reo
Rights and permissions
About this article
Cite this article
Wu, B., Li, J. & Wang, Y. Evaluation of the Microcentrifuge Dissolution Method as a Tool for Spray-Dried Dispersion. AAPS J 18, 346–353 (2016). https://doi.org/10.1208/s12248-016-9872-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-016-9872-7