Abstract
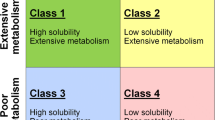
Biliary excretion is an important route of elimination for many drugs, yet measuring the extent of biliary elimination is difficult, invasive, and variable. Biliary elimination has been quantified for few drugs with a limited number of subjects, who are often diseased patients. An accurate prediction of which drugs or new molecular entities are significantly eliminated in the bile may predict potential drug-drug interactions, pharmacokinetics, and toxicities. The Biopharmaceutics Drug Disposition Classification System (BDDCS) characterizes significant routes of drug elimination, identifies potential transporter effects, and is useful in understanding drug-drug interactions. Class 1 and 2 drugs are primarily eliminated in humans via metabolism and will not exhibit significant biliary excretion of parent compound. In contrast, class 3 and 4 drugs are primarily excreted unchanged in the urine or bile. Here, we characterize the significant elimination route of 105 orally administered class 3 and 4 drugs. We introduce and validate a novel model, predicting significant biliary elimination using a simple classification scheme. The model is accurate for 83% of 30 drugs collected after model development. The model corroborates the observation that biliarily eliminated drugs have high molecular weights, while demonstrating the necessity of considering route of administration and extent of metabolism when predicting biliary excretion. Interestingly, a predictor of potential metabolism significantly improves predictions of major elimination routes of poorly metabolized drugs. This model successfully predicts the major elimination route for poorly permeable/poorly metabolized drugs and may be applied prior to human dosing.





Similar content being viewed by others
References
Grime K, Paine SW. Species differences in biliary clearance and possible relevance of hepatic uptake and efflux transporters involvement. Drug Metab Dispos. 2013;41:372–8. doi:10.1124/dmd.112.049312.
Simonson SG, Raza A, Martin PD, Mitchell PD, Jarcho JA, Brown CD, et al. Rosuvastatin pharmacokinetics in heart transplant recipients administered an antirejection regimen including cyclosporine. Clin Pharmacol Ther. 2004;76:167–77. doi:10.1016/j.clpt.2004.03.010.
Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, et al. Membrane transporters in drug development. Nat Rev Drug Discov. 2010;9:215–36. doi:10.1038/nrd3028.
Wu CY, Benet LZ. Predicting drug disposition via application of BCS: transport/absorption/elimination interplay and development of a biopharmaceutics drug disposition classification system. Pharm Res. 2005;22:11–23. doi:10.1007/s11095-004-9004-4.
Varma MVS, Chang G, Lai Y, Feng B, El-Kattan AF, Litchfield J, et al. Physicochemical property space of hepatobiliary transport and computational models for predicting rat biliary excretion. Drug Metab Dispos. 2012;40:1527–37. doi:10.1124/dmd.112.044628.
Broccatelli F. QSAR models for P-glycoprotein transport based on a highly consistent data set. J Chem Inf Model. 2012;52:2462–70. doi:10.1021/ci3002809.
Roberts MS, Magnusson BM, Burczynski FJ, Weiss M. Enterohepatic circulation: physiological, pharmacokinetic and clinical implications. Clin Pharmacokinet. 2002;41:751–90.
Watanabe T, Kusuhara H, Maeda K, Kanamaru H, Saito Y, Hu Z, et al. Investigation of the rate-determining process in the hepatic elimination of HMG-CoA reductase inhibitors in rats and humans. Drug Metab Dispos. 2010;38:215–22. doi:10.1124/dmd.109.030254.
Iwatsubo T, Hirota N, Ooie T, Suzuki H, Shimada N, Chiba K, et al. Prediction of in vivo drug metabolism in the human liver from in vitro metabolism data. Pharmacol Ther. 1997;73:147–71. doi:10.1016/S0163-7258(96)00184-2.
Obach RS. Prediction of human clearance of twenty-nine drugs from hepatic microsomal intrinsic clearance data: an examination of in vitro half-life approach and nonspecific binding to microsomes. Drug Metab Dispos. 1999;27:1350–9.
Naritomi Y, Terashita S, Kimura S, Suzuki A, Kagayama A, Sugiyama Y. Prediction of human hepatic clearance from in vivo animal experiments and in vitro metabolic studies with liver microsomes from animals and humans. Drug Metab Dispos. 2001;29:1316–24.
Stringer R, Nicklin PL, Houston JB. Reliability of human cryopreserved hepatocytes and liver microsomes as in vitro systems to predict metabolic clearance. Xenobiotica. 2008;38:1313–29. doi:10.1080/00498250802446286.
Kilford PJ, Stringer R, Sohal B, Houston JB, Galetin A. Prediction of drug clearance by glucuronidation from in vitro data: use of combined cytochrome P450 and UDP-glucuronosyltransferase cofactors in alamethicin-activated human liver microsomes. Drug Metab Dispos. 2009;37:82–9. doi:10.1124/dmd.108.023853.
Ghibellini G, Leslie EM, Brouwer KLR. Methods to evaluate biliary excretion of drugs in humans: an updated review. Mol Pharm. 2006;3:198–211. doi:10.1021/mp060011k.
LeCluyse EL, Audus KL, Hochman JH. Formation of extensive canalicular networks by rat hepatocytes cultured in collagen-sandwich configuration. Am J Physiol Cell Physiol. 1994;266:C1764–6.
Liu X, Brouwer KLR, Gan LSL, Brouwer KR, Stieger B, Meier P, et al. Partial maintenance of taurocholate uptake by adult rat hepatocytes cultured in a collagen sandwich configuration. Pharm Res. 1998;15:1533–9.
Kotani N, Maeda K, Watanabe T, Hiramatsu M, Gong LK, Bi YA, et al. Culture period-dependent changes in the uptake of transporter substrates in sandwich-cultured rat and human hepatocytes. Drug Metab Dispos. 2011;39:1503–10. doi:10.1124/dmd.111.038968.
Kusuhara H, Sugiyama Y. Pharmacokinetic modeling of the hepatobiliary transport mediated by cooperation of uptake and efflux transporters. Drug Metab Rev. 2010;42:539–50. doi:10.3109/03602530903491824.
Abe K, Bridges AS, Yue W, Brouwer KL. In vitro biliary clearance of angiotensin II receptor blockers and 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors in sandwich-cultured rat hepatocytes: comparison with in vivo biliary clearance. J Pharmacol Exp Ther. 2008;326:983–90. doi:10.1124/jpet.108.138073.
Abe K, Bridges AS, Brouwer KL. Use of sandwich-cultured human hepatocytes to predict biliary clearance of angiotensin II receptor blockers and HMG-CoA reductase inhibitors. Drug Metab Dispos. 2009;37:447–52. doi:10.1124/dmd.108.023465.
Fukuda H, Ohashi R, Tsuda-Tsukimoto M, Tamai I. Effect of plasma protein binding on in vitro-in vivo correlation of biliary excretion of drugs evaluated by sandwich-cultured rat hepatocytes. Drug Metab Dispos. 2008;36:1275–82. doi:10.1124/dmd.107.019026.
Davies B, Morris T. Physiological parameters in laboratory animals and humans. Pharm Res. 1993;10:1093–6.
Li M, Yuan H, Lin N, Song G, Zheng Y, Baratta M, et al. Identification of interspecies difference in efflux transporters of hepatocytes from dog, rat, monkey, and human. Eur J Pharm Sci. 2008;35:114–26. doi:10.1016/j.ejps.2008.06.008.
Li N, Zhang Y, Hua F, Lai Y. Absolute difference of hepatobiliary transporter multidrug resistance-associated protein (MRP2/Mrp2) in liver tissues and isolated hepatocytes from rat, dog, monkey, and human. Drug Metab Dispos. 2009;31:66–73. doi:10.1124/dmd.108.023234.
Li N, Palandra J, Nemirovskiy O, Lai Y. LC-MS/MS mediated absolute quantification and comparison of bile salt export pump and breast cancer resistance protein in livers and hepatocytes across species. Anal Chem. 2009;81:2251–9. doi:10.1021/ac8024009.
Millburn P. Factors in the biliary excretion of organic compounds. In: Fishman WH, editor. Metabolic conjugation and metabolic hydrolysis. New York: Academic; 1970. p. 1–70.
Yang X, Gandhi YA, Duignan DB, Morris ME. Prediction of biliary excretion in rats and humans using molecular weight and quantitative structure–pharmacokinetic relationships. AAPS J. 2009;11:511–25. doi:10.1208/s12248-009-9124-1.
Sharifi M, Ghafourian T. Estimation of biliary excretion of foreign compounds using properties of molecular structure. AAPS J. 2014;16:65–77. doi:10.1208/s12248-013-9541-z.
Luo G, Johnson S, Hsueh MM, Zheng J, Cai H, Xin B, et al. In silico prediction of biliary excretion of drugs based on physicochemical properties. Drug Metab Dispos. 2010;38:422–30. doi:10.1124/dmd.108.026260.
Chen Y, Cameron K, Guzman-Perez A, Perry D, Li D, Gao H. Structure-pharmacokinetic relationship of in vivo rat biliary excretion. Biopharm Drug Dispos. 2010;31:82–90. doi:10.1002/bdd.692.
Benet LZ, Broccatelli F, Oprea TI. BDDCS applied to over 900 drugs. AAPS J. 2011;13:519–47. doi:10.1208/s12248-011-9290-9.
Varma MV, Gardner I, Steyn SJ, Nkansah P, Rotter CJ, Whitney-Pickett C, et al. pH-dependent solubility and permeability criteria for provisional biopharmaceutics classification (BCS and BDDCS) in early drug discovery. Mol Pharm. 2012;9:1199–212. doi:10.1021/mp2004912.
Benet LZ. The role of BCS (biopharmaceutics classification system) and BDDCS (biopharmaceutics drug disposition classification system) in drug development. J Pharm Sci. 2013;102:34–42. doi:10.1002/jps.23359.
Larregieu CA, Benet LZ. Drug discovery and regulatiory considerations for improving in silico and in vitro predictions that use Caco-2 as a surrogate for human intestinal permeability measurements. AAPS J. 2013;15:483–97. doi:10.1208/s12248-013-9456-8.
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeablility in drug discovery and development settings. Adv Drug Deliv Rev. 1997;23:3–25. doi:10.1016/S0169-409X(96)00423-1.
VolSurf+ manual (http://www.moldiscovery.com)
Cruciani G, Crivori P, Carrupt P-A, Testa B. Molecular fields in quantitative structure-permeation relationships: the VolSurf approach. J Mol Struct. 2000;503:17–30. doi:10.1016/S0166-1280(99)00360-7.
Cruciani G, Crivori P, Carrupt P-A, Testa B. Predicting blood-brain barrier permeation from three-dimensional molecular structure. J Med Chem. 2000;43:2204–16. doi:10.1021/jm990968+.
Simulations Plus, Inc. ADMET Predictor (Version 6) {software} Retrieved from http://www.simulations-plus.com.
R Core Team. R: A language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria. 2013. Available from: http://www.R-project.org/.
Mevik B-H, Wehrens R, Liland KH. pls: partial least squares and principal component regression. 2013. R package version 2.3-0. Available from: http://CRAN.R-project.org/package=pls.
Sing T, Sander O, Beerenwinkel N, Lengauer T. ROCR: visualizing classifier performance in R. Bioinformatics. 2005;21:7881. Available from: http://rocr.bioinf.mpi-sb.mpg.de.
Demšar J, Zupan B. Orange: from experimental machine learning to interactive data mining. In: Boulicaut JF, Esposito F, Giannotti F, Pedreschi D, editors. Knowledge discovery in databases: PKDD 2004. Berlin: Springer; 2004. p. 537–9.
Zhou L, Chen X, Gu Y, Liang J. Transport characteristics of candesartan in human intestinal Caco-2 cell line. Biopharm Drug Dispos. 2009;30:259–64. doi:10.1002/bdd.664.
Salix Pharmaceuticals, Inc. Xifaxan® (rifaximin) Tablets, 550 mg. NDA 22–554. 2010. Available from: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/GastrointestinalDrugsAdvisoryCommittee/UCM201081.pdf.
Yamashiro W, Maeda K, Hirouchi M, Adachi Y, Hu Z, Sugiyama Y. Involvement of transporters in the hepatic uptake and biliary excretion of valsartan, a selective antagonist of the angiotensin II AT1-receptor, in humans. Drug Metab Dispos. 2006;34:1247–54. doi:10.1124/dmd.105.008938.
Kato Y, Miyazaki T, Kano T, Sugiura T, Kubo Y, Tsuji A. Involvement of influx and efflux transport systems in gastrointestinal absorption of celiprolol. J Pharm Sci. 2009;98:2529–39. doi:10.1002/jps.21618.
Brillault J, De Castro WV, Harnois T, Kitzis A, Olivier JC, Couet W. P-glycoprotein-mediated transport of moxifloxacin in a Calu-3 lung epithelial cell model. Antimicrob Agents Chemother. 2009;53:1457–62. doi:10.1128/AC.01253-08.
Haritova AM, Schrickx J, Lashev L, Fink-Gremmels J. ABC efflux transporters: P-gp, MRP2, and BCRP—the 3rd dimension in kinetics not only of fluoroquinolones. Br J Vet Med. 2006;9:223–42.
He XJ, Zhao LM, Qiu F, Sun YX, Li-Ling J. Influence of ABCB1 gene polymophisms on the pharmacokinetics of azithromycin among healthy Chinese Han ethnic subjects. Pharmacol Rep. 2009;61:843–50.
Deacon CF. Dipeptidyl peptidase-4 inhibitors in the treatment of type 2 diabetes: a comparative review. Diabetes Obes Metab. 2011;13:7–18. doi:10.1111/j.1463-1326.2010.01306.x.
Gladziwa U, Klotz U. Pharmacokinetics and pharmacodynamics of H2-receptor antagonists in patients with renal insufficiency. Clin Pharmacokinet. 1993;24:319–32. doi:10.2165/00003088-199324040-00005.
Hansch C, Steinmetz WE, Leo AJ, Mekapati SB, Kurup A, Hoekman D. On the role of polarizability in chemical-biological interactions. J Chem Inf Comput Sci. 2003;43:120–5. doi:10.1021/ci020378b.
Shen DD, Azarnoff DL. Clinical pharmacokinetics of methotrexate. Clin Pharmacokinet. 1978;3:1–13.
Calvert AH, Bondy PK, Harrap KR. Some observations on the human pharmacology of methotrexate. Cancer Treat Rep. 1977;61:1647–56.
Suda Y, Akazawa S. Biliary and pancreatic excretion of methotrexate (MTX) and 5-FU on the MTX/5-FU sequential therapy. Gan To Kagaku Ryoho. 1990;17:1357–63.
Nuernberg B, Koehnke R, Solsky M, Hoffman J, Furst DE. Biliary elimination of low-dose methotrexate in humans. Arthritis Rheum. 1990;33:898–902. doi:10.1002/art.1780330620.
Crivori P, Zamora I, Speed B, Orrenius C, Poggesi I. Model based on GRID-derived descriptors for estimating CYP3A4 enzyme stability of potential drug candidates. J Comput Aided Mol Des. 2004;18:155–66. doi:10.1023/B:JCAM.0000035184.11906.c2.
Masimirembwa CM, Bredberg U, Andersson TB. Metabolic stability for drug discovery and development: pharmacokinetic and biochemical challenges. Clin Pharmacokinet. 2003;42:515–28. doi:10.2165/00003088-200342060-00002.
Clarke SE, Jeffrey P. Utility of metabolic stability screening: comparison of in vitro and in vivo clearance. Xenobiotica. 2001;31:591–8. doi:10.1080/00498250110057350.
Gustafson JH, Benet LZ. Biliary excretion kinetics of phenolphthalein glucuronide after intravenous and retrograde biliary administration. J Pharm Pharmacol. 1974;26:937–44. doi:10.1111/j.2042-7158.1974.tb09212.x.
Wacher VJ, Wu C-Y, Benet LZ. Overlapping substrate specificities and tissue distribution of cytochrome P450 3A and P-glycoprotein: implications for drug delivery and activity in cancer chemotherapy. Mol Carcinog. 1995;13:129–34. doi:10.1002/mc.2940130302.
Hansch C, Kurup A. QSAR of chemical polarizability and nerve toxicity. 2. J Chem Inf Comput Sci. 2003;43:1647–51.
Quillin ML, Breyer WA, Griswold IJ, Matthews BW. Size versus polarizability in protein-ligand interactions: binding of noble gases within engineered cavities in phage T4 lysozyme. J Mol Biol. 2000;302:955–77. doi:10.1006/jmbi.2000.4063.
Chacra AR. Saxagliptin for type 2 diabetes. Diabetes Metab Syndr Obes. 2010;3:325–35. doi:10.2147/DMSOTT.S12241.
Renkin EM, Robinson RR. Glomerular filtration. N Engl J Med. 1974;290:785–92.
Acknowledgments
We would like to thank Dr. Julian Blagg at the Institute of Cancer Research for critical review of the manuscript, Dr. Gabriele Cruciani and Molecular Discovery Ltd. for generously supplying the VolSurf+ license, and Dr. Michael Bolger and Simulations Plus for generously supplying the ADMET Predictor™ license. Chelsea Hosey was supported in part by NIH Training Grant T32 GM007175.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hosey, C.M., Broccatelli, F. & Benet, L.Z. Predicting when Biliary Excretion of Parent Drug is a Major Route of Elimination in Humans. AAPS J 16, 1085–1096 (2014). https://doi.org/10.1208/s12248-014-9636-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-014-9636-1