Abstract
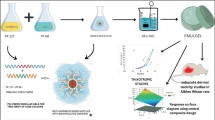
The current study is concerned with the development and characterization of mixed micelles intended for the dermal delivery of beclomethasone dipropionate, which is a topical corticosteroid used in the management of atopic dermatitis. Mixed micelles were prepared using thin-film hydration technique, employing different concentrations of pluronic L121 with either poloxamer P84 or pluronic F127 with different surfactant mixture-to-drug ratios. The prepared formulae were characterized concerning entrapment efficiency, particle size, and zeta potential. Two formulae were chosen for ex vivo skin deposition studies: one formulated using pluronic L121/poloxamer P84 mixture while the other using pluronic L121/pluronic F127 mixture. The optimum formula with the highest dermal deposition was subjected to morphological examination and was formulated as hydroxypropyl methylcellulose hydrogel. The hydrogel was evaluated regarding viscosity and was subjected to ex vivo deposition study in comparison with the commercially available cream Beclozone®. In vivo histopathological study was conducted for both the hydrogel and Beclozone® in order to evaluate their healing efficiency. In vivo histopathological study results showed that the prepared hydrogel successfully treated sub-chronic dermatitis in an animal model within a shorter period of time compared to Beclozone®, resulting in better patient compliance and fewer side effects.



Similar content being viewed by others
References
Dai J, Choo MK, Park JM, Fisher DE. Topical ROR inverse agonists suppress inflammation in mouse models of atopic dermatitis and acute irritant dermatitis. J Invest Dermatol. 2017;137(12):2523–31.
Nida Akhtar AV, Pathak K. Exploring preclinical and clinical effectiveness of nanoformulations in the treatment of atopic dermatitis: safety aspects and patent review. Bull Facult Pharm Cairo Univ. 2016;55(1):1–10.
Jung DL, Lee SD, Choi IH, Na HS, Hong SU. Effects of electroacupuncture on capsaicin-induced model of atopic dermatitis in rats. J Dermatol Sci. 2014;74(1):23–30.
LeBovidge JS, Elverson W, Timmons KG, Hawryluk EB, Rea C, Lee M, et al. Multidisciplinary interventions in the management of atopic dermatitis. J Allergy Clin Immunol. 2016;138(2):325–34.
Noguchi A, Tominaga M, Takahashi N, Matsuda H, Kamata Y, Umehara Y, et al. Differences in therapeutic effects of topically applied corticosteroid and tacrolimus on atopic dermatitis-like symptoms in NC/Nga mice. J Dermatol Sci. 2017;86(1):54–62.
Baboota S, Alam MS, Sharma S, Sahni JK, Kumar A, Ali J. Nanocarrier-based hydrogel of betamethasone dipropionate and salicylic acid for treatment of psoriasis. Int J Pharm Investig. 2011;1(3):139–47.
Aliabadi HM, Lavasanifar A. Polymeric micelles for drug delivery. Expert Opin Drug deliv. 2006;3(1):139–62.
Panja SK. Therapeutic update of eczema and topical corticosteroids. Indian J Dermatol. 1990;35(1):1–15.
Lapteva M, Moller M, Gurny R, Kalia YN. Self-assembled polymeric nanocarriers for the targeted delivery of retinoic acid to the hair follicle. Nanoscale. 2015;7(44):18651–62.
Lapteva M, Santer V, Mondon K, Patmanidis I, Chiriano G, Scapozza L, et al. Targeted cutaneous delivery of ciclosporin A using micellar nanocarriers and the possible role of inter-cluster regions as molecular transport pathways. J Control Release. 2014;196:9–18.
Morsi NM, Abdelbary GA, Ahmed MA. Silver sulfadiazine based cubosome hydrogels for topical treatment of burns: development and in vitro/in vivo characterization. Eur J Pharm Biopharm. 2014;86(2):178–89.
Kassem MAA, ElMeshad AN, Fares AR. Enhanced solubility and dissolution rate of lacidipine nanosuspension: formulation via antisolvent sonoprecipitation technique and optimization using Box–Behnken design. AAPS PharmSciTech. 2017;18(4):983–96.
Newcomer CE. The evolution and adoption of standards used by AAALAC. J Am Assoc Lab Anim Sci. 2012;51(3):293–7.
Radwan SAA, ElMeshad AN, Shoukri RA. Microemulsion loaded hydrogel as a promising vehicle for dermal delivery of the antifungal sertaconazole: design, optimization and ex vivo evaluation. Drug Dev Ind Pharm. 2017;43(8):1351–65.
Reid ML, Benaouda F, Khengar R, Jones SA, Brown MB. Topical corticosteroid delivery into human skin using hydrofluoroalkane metered dose aerosol sprays. Int J Pharm. 2013;452(1–2):157–65.
De Leo V, Ruscigno S, Trapani A, Di Gioia S, Milano F, Mandracchia D, et al. Preparation of drug-loaded small unilamellar liposomes and evaluation of their potential for the treatment of chronic respiratory diseases. Int J Pharm. 2018;545(1–2):378–88.
Al-Mahallawi AM, Khowessah OM, Shoukri RA. Nano-transfersomal ciprofloxacin loaded vesicles for non-invasive trans-tympanic ototopical delivery: in-vitro optimization, ex-vivo permeation studies, and in-vivo assessment. Int J Pharm. 2014;472(1–2):304–14.
Agubata CO, Okereke C, Nzekwe IT, Onoja RI, Obitte NC. Development and evaluation of wound healing hydrogels based on a quinolone, hydroxypropyl methylcellulose and biodegradable microfibres. Eur J Pharm Sci. 2016;89:1–10.
Tung I-C. Rheological behavior of poloxamer 407 aqueous solutions during sol–gel and dehydration processes. Int J Pharm. 1994;107:85–90.
Gupta AK, Fisher GJ, Elder JT, Talwar HS, Esmann J, Duell EA, et al. Topical cyclosporine a inhibits the phorbol ester induced hyperplastic inflammatory response but not protein kinase C activation in mouse epidermis. J Investig Dermatol. 1989;93(3):379–86.
Hernández-Valle E, Herrera-Ruiz M, Salgado GR, Zamilpa A, Ocampo ML, Aparicio AJ, et al. Anti-inflammatory effect of 3-O-[(6′-O-palmitoyl)-β-D-glucopyranosyl sitosterol] from Agave angustifolia on ear edema in mice. Molecules. 2014;19:15624–37.
John D, Bancroft AS. Theory and practice of histological techniques. 4th ed. New York: Churchill Livingstone; 1996.
Caddeo C, Sales OD, Valenti D, Sauri AR, Fadda AM, Manconi M. Inhibition of skin inflammation in mice by diclofenac in vesicular carriers: liposomes, ethosomes and PEVs. Int J Pharm. 2013;443(1–2):128–36.
Jindal N, Mehta SK. Nevirapine loaded Poloxamer 407/Pluronic P123 mixed micelles: optimization of formulation and in vitro evaluation. Colloids Surf B: Biointerfaces. 2015;129:100–6.
Lee ES, Oh YT, Youn YS, Nam M, Park B, Yun J, et al. Binary mixing of micelles using Pluronics for a nano-sized drug delivery system. Colloids Surf B: Biointerfaces. 2011;82(1):190–5.
Abdelbary GA, Tadros MI. Brain targeting of olanzapine via intranasal delivery of core–shell difunctional block copolymer mixed nanomicellar carriers: in vitro characterization, ex vivo estimation of nasal toxicity and in vivo biodistribution studies. Int J Pharm. 2013;452(1–2):300–10.
Chiappetta DA, Sosnik A. Poly(ethylene oxide)-poly(propylene oxide) block copolymer micelles as drug delivery agents: improved hydrosolubility, stability and bioavailability of drugs. Eur J Pharm Biopharmaceutics. 2007;66(3):303–17.
Singla P, Chabba S, Mahajan RK. A systematic physicochemical investigation on solubilization and in vitro release of poorly water soluble oxcarbazepine drug in pluronic micelles. Colloids Surf A Physicochem Eng Asp. 2016;504:479–88.
Salama AH, Shamma RN. Tri/tetra-block co-polymeric nanocarriers as a potential ocular delivery system of lornoxicam: in-vitro characterization, and in-vivo estimation of corneal permeation. Int J Pharm. 2015;492(1–2):28–39.
Petrov PDGG, Gancheva V, Trusheva B, Bankova V, Tsvetanov CB. Development of propolis-loaded block copolymer micelles of superior structural stability and high loading capacity. Polymer. 2017;125:102–9.
Kadam Y, Yerramilli U, Bahadur A, Bahadur P. Micelles from PEO-PPO-PEO block copolymers as nanocontainers for solubilization of a poorly water soluble drug hydrochlorothiazide. Colloids Surf B: Biointerfaces. 2011;83(1):49–57.
El-Dahmy RM, Elsayed I, Elshafeey AH, Gawad NA, El-Gazayerly ON. Optimization of long circulating mixed polymeric micelles containing vinpocetine using simple lattice mixture design, in vitro and in vivo characterization. Int J Pharm. 2014;477(1–2):39–46.
Kim S, Shi Y, Kim JY, Park K, Cheng JX. Overcoming the barriers in micellar drug delivery: loading efficiency, in vivo stability, and micelle–cell interaction. Expert Opin Drug Deliv. 2010;7(1):49–62.
Pepic I, Lovric J, Hafner A, Filipovic-Grcic J. Powder form and stability of Pluronic mixed micelle dispersions for drug delivery applications. Drug Dev Ind Pharm. 2014;40(7):944–51.
Zhao L, Du J, Duan Y, Zang Y, Zhang H, Yang C, et al. Curcumin loaded mixed micelles composed of Pluronic P123 and F68: preparation, optimization and in vitro characterization. Colloids Surf B: Biointerfaces. 2012;97:101–8.
Nour SA, Abdelmalak NS, Naguib MJ, Rashed HM, Ibrahim AB. Intranasal brain-targeted clonazepam polymeric micelles for immediate control of status epilepticus: in vitro optimization, ex vivo determination of cytotoxicity, in vivo biodistribution and pharmacodynamics studies. Drug Deliv. 2016;23(9):3681–95.
Ebrahim Attia AB, Ong ZY, Hedrick JL, Lee PP, Ee PLR, Hammond PT, Yang Y-Y. Mixed micelles self-assembled from block copolymers for drug delivery. Curr Opin Colloid Interface Sci. 2011;16:182–94.
Zhang H, Zhao L, Chu L, Han X, Zhai G. Preparation, optimization, characterization and cytotoxicity in vitro of Baicalin-loaded mixed micelles. J Colloid Interface Sci. 2014;434:40–7.
Wei Z, Hao J, Yuan S, Li Y, Juan W, Sha X, et al. Paclitaxel-loaded Pluronic P123/F127 mixed polymeric micelles: formulation, optimization and in vitro characterization. Int J Pharm. 2009;376(1–2):176–85.
Marianecci C, Paolino D, Celia C, Fresta M, Carafa M, Alhaique F. Non-ionic surfactant vesicles in pulmonary glucocorticoid delivery: characterization and interaction with human lung fibroblasts. J Control Release. 2010;147(1):127–35.
Duan Y, Cai X, Du H, Zhai G. Novel in situ gel systems based on P123/TPGS mixed micelles and gellan gum for ophthalmic delivery of curcumin. Colloids Surf B: Biointerfaces. 2015;128:322–30.
Kulthe SS, Inamdar NN, Choudhari YM, Shirolikar SM, Borde LC, Mourya VK. Mixed micelle formation with hydrophobic and hydrophilic Pluronic block copolymers: implications for controlled and targeted drug delivery. Colloids Surf B: Biointerfaces. 2011;88(2):691–6.
Abdelbary AA, Li X, El-Nabarawi M, Elassasy A, Jasti B. Effect of fixed aqueous layer thickness of polymeric stabilizers on zeta potential and stability of aripiprazole nanosuspensions. Pharm Dev Technol. 2013;18(3):730–5.
Maria Lapteva KM, Möller M, Gurny R, Kalia YN. Polymeric micelle nanocarriers for the cutaneous delivery of tacrolimus: a targeted approach for the treatment of psoriasis. Mol Pharm. 2014;11:2989–3001.
Bachhav YG, Mondon K, Kalia YN, Gurny R, Moller M. Novel micelle formulations to increase cutaneous bioavailability of azole antifungals. J Control Release. 2011;153(2):126–32.
Acknowledgments
The authors would like to gratefully praise the great efforts exerted by Assistant Prof. Dr. Ahmed R. Fares in the statistical part and the organization of the whole manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Assem, M., Khowessah, O.M. & Ghorab, D. Optimization and Evaluation of Beclomethasone Dipropionate Micelles Incorporated into Biocompatible Hydrogel Using a Sub-Chronic Dermatitis Animal Model. AAPS PharmSciTech 20, 152 (2019). https://doi.org/10.1208/s12249-019-1355-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-019-1355-6