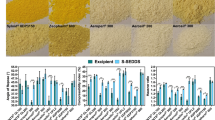
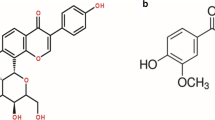
Abstract
The manufacture of personalised medicines where specific combinations of active pharmaceutical ingredients (APIs) and their dose within a tablet would be adjusted to the needs of individual patients, would require new manufacturing approaches compared to the established practice. In the case of low-dose formulations, the required precision of API content might not be achievable by traditional unit operations such as solid powder blending. The aim of the present work was to explore an alternative approach, based on the concept of pre-formulated placebo tablets containing mesoporous silica particles capable of absorbing APIs in the form of solutions, which can be precisely dosed at arbitrarily low quantities. The precision of the liquid dosing system has been validated; it was shown that the mechanical properties of the tablets were satisfactory even after multiple impregnation-drying cycles and that pharmacopoeia specifications on content uniformity could be met. Using model APIs, the spatial distribution of the API within the tablet after impregnation was investigated and shown to depend on the number and order of the impregnation-drying cycles. It was found that when an API was loaded to the tablet in a single step, a different dissolution profile was obtained compared to the same quantity dosed in multiple smaller steps. Overall, the approach of loading multiple API to a pre-formulated tablet at defined quantities using drop-on-demand liquid dosing was found to be feasible from the dose uniformity point of view. Further research should focus on potential API interactions and storage stability of tablets manufactured in this way.










Similar content being viewed by others
References
Anaya J-M, Duarte-Rey C, Sarmiento-Monroy JC, Bardey D, Castiblanco J, Rojas-Villarraga A. Personalized medicine. Closing the gap between knowledge and clinical practice. Autoimmun Rev. 2016;15:833–42. https://doi.org/10.1016/j.autrev.2016.06.005.
Herxheimer A. How much drug in the tablet? Lancet. 1991;337:346–348. https://doi.org/10.1016/0140-6736(91)90958-R.
Lind J, Sporrong SK, Kaae S, Rantanen J, Genina N. Social aspects in additive manufacturing of pharmaceutical products. Expert Opin. Drug Deliv. 2017;14:927–36. https://doi.org/10.1080/17425247.2017.1266336.
Alomari M, Mohamed FH, Basit AW, Gaisford S. Personalised dosing: printing a dose of one’s own medicine. Int J Pharm. 2015;494:568–77. https://doi.org/10.1016/j.ijpharm.2014.12.006.
Scoutaris N, Alexander MR, Gellert PR, Roberts CJ. Inkjet printing as a novel medicine formulation technique. J Control Release. 2011;156:179–85. https://doi.org/10.1016/j.jconrel.2011.07.033.
Genina N, Fors D, Palo M, Peltonen J, Sandler N. Behavior of printable formulations of loperamide and caffeine on different substrates—effect of print density in inkjet printing. Int J Pharm. 2013;453:488–97. https://doi.org/10.1016/j.ijpharm.2013.06.003.
Pardeike J, Strohmeier DM, Schrödl N, Voura C, Gruber M, Khinast JG, et al. Nanosuspensions as advanced printing ink for accurate dosing of poorly soluble drugs in personalized medicines. Int J Pharm. 2011;420:93–100. https://doi.org/10.1016/j.ijpharm.2011.08.033.
Scoutaris N, Ross S, Douroumis D. Current trends on medical and pharmaceutical applications of inkjet printing technology. Pharm Res. 2016;33:1799–816. https://doi.org/10.1007/s11095-016-1931-3.
Sandler N, Määttänen A, Ihalainen P, Kronberg L, Meierjohann A, Viitala T, et al. Inkjet printing of drug substances and use of porous substrates—towards individualized dosing. J Pharm Sci. 2011;100:3386–95. https://doi.org/10.1002/JPS.22526.
Kollamaram G, Hopkins SC, Glowacki BA, Croker DM, Walker GM. Inkjet printing of paracetamol and indomethacin using electromagnetic technology: rheological compatibility and polymorphic selectivity. Eur J Pharm Sci. 2018;115:248–57. https://doi.org/10.1016/J.EJPS.2018.01.036.
Bonhoeffer B, Kwade A, Juhnke M. Alternative manufacturing concepts for solid oral dosage forms from drug nanosuspensions using fluid dispensing and forced drying technology. J Pharm Sci. 2018;107:909–21. https://doi.org/10.1016/J.XPHS.2017.11.007.
Hirshfield L, Giridhar A, Taylor LS, Harris MT, Reklaitis GV. Dropwise additive manufacturing of pharmaceutical products for solvent-based dosage forms. J Pharm Sci. 2014;103:496–506. https://doi.org/10.1002/JPS.23803.
Içten E, Giridhar A, Taylor LS, Nagy ZK, Reklaitis GV. Dropwise additive manufacturing of pharmaceutical products for melt-based dosage forms. J Pharm Sci. 2015;104:1641–9. https://doi.org/10.1002/JPS.24367.
Içten E, Purohit HS, Wallace C, Giridhar A, Taylor LS, Nagy ZK, et al. Dropwise additive manufacturing of pharmaceutical products for amorphous and self emulsifying drug delivery systems. Int J Pharm. 2017;524:424–32. https://doi.org/10.1016/J.IJPHARM.2017.04.003.
Šoltys M, Kovačík P, Dammer O, Beránek J, Štěpánek F. Effect of solvent selection on drug loading and amorphisation in mesoporous silica particles. Int J Pharm. 2019;555:19–27. https://doi.org/10.1016/j.ijpharm.2018.10.075.
Van Speybroeck M, Barillaro V, Do Thi T, Mellaerts R, Martens J, Van Humbeeck J, et al. Ordered mesoporous silica material SBA-15: a broad-spectrum formulation platform for poorly soluble drugs. J Pharm Sci. 2009;98:2648–58. https://doi.org/10.1002/jps.21638.
Limnell T, Santos HA, Mäkilä E, Heikkilä T, Salonen J, Murzin DY, et al. Drug delivery formulations of ordered and nonordered mesoporous silica: comparison of three drug loading methods. J Pharm Sci. 2011;100:3294–306. https://doi.org/10.1002/jps.22577.
McCarthy CA, Ahern RJ, Dontireddy R, Ryan KB, Crean AM. Mesoporous silica formulation strategies for drug dissolution enhancement: a review. Expert Opin Drug Deliv. 2016;13:93–108. https://doi.org/10.1517/17425247.2016.1100165.
Teng Z, Han Y, Li J, Yan F, Yang W. Preparation of hollow mesoporous silica spheres by a sol–gel/emulsion approach. Microporous Mesoporous Mater. 2010;127:67–72. https://doi.org/10.1016/j.micromeso.2009.06.028.
Šoltys M, Balouch M, Kašpar O, Lhotka M, Ulbrich P, Zadražil A, et al. Evaluation of scale-up strategies for the batch synthesis of dense and hollow mesoporous silica microspheres. Chem Eng J. 2018;334:1135–47. https://doi.org/10.1016/j.cej.2017.11.026.
Council of Europe. European pharmacopoeia, 6th ed. Strasbourg; 2007.
Kataria A, Oka S, Smrčka D, Grof Z, Štěpánek F, Ramachandran R. A quantitative analysis of drug migration during granule drying. Chem Eng Res Des. 2018;136:199–206. https://doi.org/10.1016/J.CHERD.2018.05.001.
Novák V, Kočí P, Štěpánek F, Kubíček M, Marek M. Simulated preparation of supported porous catalyst and evaluation of its reaction-transport properties. Comput Chem Eng. 2011;35:964–72. https://doi.org/10.1016/J.COMPCHEMENG.2011.01.039.
Kohout M, Grof Z, Štěpánek F. Pore-scale modelling and tomographic visualisation of drying in granular media. J Colloid Interface Sci. 2006;299:342–51. https://doi.org/10.1016/J.JCIS.2006.01.074.
Lizoňová D, Mužík J, Šoltys M, Beránek J, Kazarian SG, Štěpánek F. Molecular-level insight into hot-melt loading and drug release from mesoporous silica carriers. Eur J Pharm Biopharm. 2018;130:327–35. https://doi.org/10.1016/j.ejpb.2018.07.013.
Acknowledgments
This work was supported by Specific University Research (MSMT no. SVV-21/2018). We would like to thank Dr. Pavel Ulbrich for help with Transmission Electron Microscopy. Financial support from the Grant Agency of the Czech Republic (project GACR no. 16-12291S) is gratefully acknowledged. We would like to thank Zentiva, k.s., for kindly donating excipients for this work and providing access to a tablet press.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editors: Niklas Sandler and Jukka Rantanen
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Šoltys, M., Akhlasová, S., Zadražil, A. et al. Manufacturing of Multi-drug Formulations with Customised Dose by Solvent Impregnation of Mesoporous Silica Tablets. AAPS PharmSciTech 20, 25 (2019). https://doi.org/10.1208/s12249-018-1224-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-018-1224-8