Abstract
Background
Primary tumour biology and axillary lymph node status are key prognostic factors in breast cancer treatment. The LowMag trial introduced a magnetic sentinel lymph node biopsy procedure using a superparamagnetic iron oxide (SPIO) tracer and a handheld magnetometer as a radiation-free alternative for axillary staging. The trial aimed to assess a low-dose magnetic tracer for non-invasive preoperative evaluation of lymph node metastases and intraoperative sentinel lymph node detection.
Methods
Patients with confirmed invasive breast cancer or ductal carcinoma in situ, eligible for sentinel lymph node biopsy, were included in the LowMag trial. The sentinel lymph nodes were detected perioperatively using Sentimag® and inked to maintain spatial orientation between MRI and histopathology. The amount of iron was estimated using two magnetic devices: SPaQ and Sentimag®. Additional ex vivo MRI was performed with a low-field MRI system. After being buffered in formalin, the sentinel lymph nodes were sectioned perpendicular to the MRI planes, consecutively sliced at 2 μm intervals, and stained with H&E, Perls Prussian blue, CK8/18, and CD68.
Results
In an interim assessment of 20 sentinel lymph nodes, two contained metastases. The analysis revealed good concordance in uptake between the magnetic and radioactive tracers, with a median iron content of 19.21 µg. In healthy sentinel lymph nodes, iron particles were found in both the subcapsular space and sinusoids, with macrophages nearby. Healthy regions within metastatic lymph nodes showed similar behaviour to healthy nodes. In metastatic sites, iron pigment presence was reduced, especially in areas occupied by tumour cells. A healthy lymph node with low iron content displayed a large central fatty region without iron uptake but confirmed iron in sinusoidal macrophages. The metastatic lymph node had subcapsular and parenchymal tumour cells in the central region, with limited infiltration into nearby adipose tissue and no local iron enhancement. However, iron deposits were detected within the sinusoids.
Discussion
The MRI images effectively distinguish between fat, nodal tissue, and the SPIO tracer, either through signal intensity or texture. This demonstrates the potential for sentinel lymph node imaging using a low-field MRI system.
Similar content being viewed by others
Introduction
Primary tumour biology and axillary lymph node status are important factors when it comes to prognosis and therapeutic management in breast cancer treatment [1]. Sentinel lymph node biopsy (SLNB) with the dual technique (radioisotope and blue dye) is considered the standard method of axillary staging, both in prior surgery and after neoadjuvant treatment (NAT).
However, the combined method still suffers from disadvantages, such as the use of radioactive materials and the fact that node-positive patients require multiple surgical procedures. Although the radioactive dose is low, the requirement on radioactive tracers leads to complicated logistics and regulations and makes that the sentinel node procedure is out of reach for patients in less developed countries. Besides, for axillary SLNB there is a minor risk of treatment related morbidity, as lymphedema, limitations in shoulder range of motion, loss of strength, numbness, dysesthesias and pain. A major disadvantage especially for the 80% node-negative patients after histopathological examination [2].
In the conventional SLNB-procedure patients receive standard radioisotope injection 3–24 h preoperatively: 2 injections of Tc-99 m albumin colloid with a total activity of approximately 50–140 MBq (depending on the time frame). Two hours post-injection of the Tc99-m Nano colloid, conventional planar lymphoscintigraphy is being performed. Blue dye is only administered in case of insufficient transcutaneous signal measured pre-surgery with help of the gamma probe.
A magnetic technique, using a Superparamagnetic Iron Oxide (SPIO) tracer and handheld magnetometer for a radiation free SLNB-procedure was developed and have successfully been investigated in earlier studies to overcome drawbacks associated with the use of radioactivity [3, 4].
However, accurate imaging in the diagnostic phase could save axillary surgery in 80% of patients without lymph node metastases. Besides, Patients with lymph node metastases (20%) can undergo targeted minimally invasive axillary treatment. Preoperative stratification of patients with tumour-negative axillary nodes, or axillary nodes with a low tumour load that does not warrant further surgery (< 2 mm) but guides therapeutic decisions, may allow for a tailored approach without SLNB. Donker et al. [5] in this case, the contrast will be taken up by the lymphatic vessels, reach the SLNs and then be visualized by a radiological modality.
Harinsinghani et al. [6] were the first to demonstrate that intravenously administered Ultrasmall Superparamagnetic Iron Oxide (USPIO) enhanced Magnetic Resonance Imaging (MRI) can be used to non-invasively predict the nodal status of prostate cancer patients. USPIO and Superparamagnetic Iron Oxide (SPIO) particles exert negative contrast on MRI. The uptake of the (U)SPIO particles by macrophages that accumulate in lymph nodes, results in black-appearing nodes on MR-images. In malignancy-baring regions of the lymph node, no macrophages are accumulated and the specific regions keep their original intensity [7]. If imaging can be performed with sufficient resolution, the inhomogeneous reduction of signal in metastatic lymph nodes as compared to the homogenous reduction of signal in nonmetastatic nodes, theoretically allows for the non-invasive preoperative detection of lymph node metastases.
The objective of this study was to assess a low dose magnetic tracer for non-invasive pre-operative evaluation of lymph node metastases (Fig. 1) and intra operative SLN detection.
Future perspective. Patients with histologically proven breast cancer without clinically suspicious lymph nodes are eligible for a pre-operative SPIO injection and MRI. In case of homogeneous iron distribution, the lymph nodes are not suspicious for metastases and no further axillary treatment is needed. In case of heterogeneous iron distribution, targeted axillar treatment can be performed depending on the number of suspicious lymph nodes (i.e. radiation therapy, systemic therapy or surgery.)
Methods
Patients and trial design
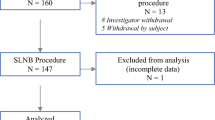
Patients with histologically confirmed invasive breast cancer or ductal carcinoma in situ (DCIS) visible on ultrasound imaging, presented to Medisch Spectrum Twente between August 2021 and August 2023, were considered for the Lowmag clinical trial (TRN NL4713, http://www.onderzoekmetmensen.nl, approved 22–10-2014). Patients eligible for SLNB [8] (cN0, clinically no metastases in the lymph nodes) and patients with cN1 (proven metastasis after biopsy) undergoing upfront surgery (lumpectomy or mastectomy) and available for a minimum of 12 months follow-up were included in the study. Patients who are allergic or hypersensitive to iron or dextran compounds were excluded. Additional patients who received neo-adjuvant treatment were excluded for participation, because the effect of NAC on SPIO uptake in the lymph nodes is in this stage unclear. Ethics Committee (Medical Research Ethics Committees United, MEC-U Nieuwegein, the Netherlands) approval was obtained before the start of inclusion, and all patients provided written informed consent.
The LowMag trial, investigates the intraoperative detectability of the SLN using a low dose peritumoural SPIO (Magtrace) injection in combination with the SentiMag detection system. Moreover, SPIO-enhanced sequences are being explored to visualize metastases in ex vivo SLN (summarised in Fig. 2). The relevant MRI sequences and processing algorithms are assessed by correlating histopathology with MR images.
An interim analysis was conducted after 20 nodes, to ensure that the Pathology and MRI protocols would provide sufficient information and quality for further correlation testing.
Prior to surgery
SPIO injection
The patients receive a peritumourally injection (Fig. 3) of 1 mL (28 mg iron) SPIO (Magtrace®, Endomag, Cambridge, UK) within a week prior to surgery. The peritumoural dorsal injection of the SPIO tracer for a magnetic SLNB is placed under Ultrasound guidance by an experienced breast radiologist. The dorsal placement is important to prevent for Susceptibility Artifacts during follow-up MRI, caused by iron remnants [9].
99mTc injection and lymphoscintigraphy
In the first study cohort, patients received a standard of care radioisotope injection 3–24 h preoperatively: as per site protocol, 2 subareolar/subcutaneous injections of Tc-99 m nanocolloid with a total activity of max 140 MBq. The injection site, either subareolar or subcutaneous, is determined by the discretion of the nuclear physician administering the injection. Two hours after the tracer injection a conventional planar lymphoscintigraphy was performed. In the second cohort the radioactive tracer was omitted.
During the surgery
Sentinel lymph node detection
The SLNs were perioperatively detected by the CE-certified handheld magnetic probe (Sentimag®, Endomagnetics, Cambridge, UK) [10] Fig. 4a). Disposable (plastic) surgical instruments were used during the magnetometer measurements, to prevent for disturbance of the magnetic field. Current-standard-of-care SLNB procedure (involving radioactive tracer and blue dye) was used in case of an unsuccessful magnetic procedure, if no hotspot can be found, or as a control measurement. One milliliter of blue dye (Patent Blue V, Laboratoire Guerbet, France) was administered subareolarly immediately following the induction of anesthesia. According to the site protocol, if a high radioactive signal was detected transcutaneously in patients, the blue dye was withheld. The magnetometer and gamma counts were documented for all excised sentinel node(s). The residual counts in the axillary region were documented for both the magnetometer and the gamma probe.
Tissue ink for lymph node orientation
Each node was inked with three colours to mark the orientation for correlation between pathology images and MR images. As illustrated in Fig. 5, a half of the node was inked with black colour. Of the remaining half, one part was inked green, and the remaining quarter was inked yellow (The Davidson Marking System ®, Bradley Products, Inc).
After the surgery
Two different devices were used to assess the iron-distribution around the excised LN.
Assessment of iron content by SPAQ
The Super Paramagnetic Quantifier (SPaQ) is an in-house developed magnetometer featuring a sample holder situated within a uniform magnetic field [11] (Fig. 6). SPaQ was employed in its differential magnetometry (DiffMag) mode [12]. The SLN (situated in a glass tube) was placed in an alternating magnetic field generated by an excitation coil to measure the dynamic magnetisation curve. The specific SPaQ settings used involve AC amplitude of 0.83 mT, AC frequency of 2.5 kHz and DC field strength of 4.99 mT [13]. The evaluation was supported by a lookup table (LUT) tailored to quantify the accumulation of the Magtrace magnetic tracer within LN through the utilisation of magnetic readout counts [14].
Assessment of iron content by Sentimag
The Sentimag magnetometer operates on the basis of magnetic susceptometry, generating a brief alternating magnetic field that temporarily magnetizes the iron oxide particles present in Magtrace. This magnetic signature, generated by the small amounts of iron, is subsequently detected by the same probe. The magnetic readout counts produced by the Sentimag magnetometer were correlated with the quantity of iron captured within the lymph node (LN) using a specifically tailored Look Up Table (LUT) designed for the Magtrace magnetic tracerFootnote 1 [15]. The counts, and consequently the trapped amount of iron, were recorded in a fixed setup of the Sentimag probe (Fig. 4B). The measurements are taken at six distinct orthogonal positions on the surface of the lymph node for each sensitivity setting (1, 2, 3) of the Sentimag as illustrated in Fig. 7. Disposable (plastic) surgical instruments were used during the magnetometer measurements, to prevent for disturbance of the magnetic field.
Ex vivo low-field MRI
The LNs were subsequently imaged using a portable low-field MRI system (Pure Devices GmbH, Rimpar, Germany) with an increased bore of 15 mm diameter at a field strength of 0.5 T (Fig. 8). The 3D spin-echo images were acquired with a field-of-view 14 × 14x14 mm and an isotropic resolution of 125 μm: Spin echo T1-weighted (T1W), T2-weighted (T2W), short tau inversion recovery (STIR), T1 and T2 maps (Table 1). Photographs were taken of the LN orientation to ensure comparison with histopathological images. As formalin is the constant element in all MRI images, we have used this to normalise weighted MRI. The MRI scan protocol was developed by a study group consisting of 2 radiologists, an MRI technician, a technical physician, and an associate professor from the Magnetic Detection and Imaging department at the University of Twente. The MRI scans were conducted by a technical physician.
Histopathology examination
After buffered in formalin fixation, the three-coloured LN were lamellated at 250 μm distance perpendicular to the MRI planes and enclosed in a cassette for further histopathological processing. Each cassette potentially contained more than one lamella. The embedded lamella was sectioned with the microtome at 2 μm distance and stained with H&E, Perls Prussian blue, CK8/18 and CD68. Figure 9 shows an overview of this procedure. The slides are scanned with Phillips' IntelliSite Ultra-Fast Scanner. For the histopathological analysis of sentinel nodes, each harvested LN was assessed on presence of iron, fat, macrophages and metastases by an experienced pathologist using a semiquantitative quaternary (0–3 +) scale, resulting in a pT stage.
In the histopathology images, the pathologist conducted a comparative analysis across all four stains for a number of relevant regions of interest (ROIs). The focus was on establishing correlations between macrophages (CD68 stain), metastases (CK8-18 marker), and iron accumulation (Perl’s Prussian Blue stain). Particular emphasis was on evaluating disparities between metastatic and non-metastatic regions within LNs containing metastases, as well as distinctions between non-metastatic regions in LNs with and without metastases.
Results
Patients and magnetic SLNB
In an interim assessment, the initial nine patients undergoing the extensive histopathology and MRI protocol were analyzed with the intention of refining the protocol. All nine participants were female with a mean age of 59.6 years (52–68). They received a peritumoural injection on either the latero cranial or dorsal side of the tumour. The average duration between injection and resection was 3.2 days, ranging from 1 to 8 days.
Sentinel lymph node detection
Two surgeons performed the SLNB procedures. A total of 20 LN was resected, with the number per patient ranging from one to five. Table 2 illustrates patient characteristics including number of LN resected and corresponding amount of iron trapped. Most of the lymph nodes (n = 12) had an uptake of both the magnetic and radioactive tracer. Three lymph nodes had uptake of only the magnetic tracer and one lymph node exclusively absorbed the radioactive tracer (Fig. 10). Fifteen out of the twenty nodes showed both magnetic and radioactive uptake. The concordance rate thereby stands at 75%.
Eight out of nine procedures could be performed using only the magnetic SLNB procedure. In one case, the radioactive procedure was used as a backup. Transcutaneous detection of the magnetic tracer was inadequate in almost all cases, once the incision was made through the skin, adequate signals were detected.
Assessment of iron content
Using SPaQ
We found median amount of iron over all 20 LN of 19.21 µg (ranging from 0.1 pg to 109 µg). On average, SLNs contained 0,1% iron of the total injected dose (1 ml = 28 mg) ranging up to 0.39%. The iron content in the metastatic LN was non-significantly higher than iron content in non-metastatic LN (Fig. 11-A).
A Spread of Iron distribution over all 20 lymph nodes, divided voor SLNs with metastases (red) and healthy SLNs (yellow) measured with SPaQ. B The spread of iron content within a specific lymph node measured with Sentimag at the six different positions. The figure illustrates the spread in a lymph node with metastasis (blue) and a lymph node without metastases (gray). C The spread of iron content in the lymph nodes is visualized for Sentimag sensitivity setting 1 (purple) 2 (orange) en 3 (brown). In de green box the iron content calculated using SPaQ is shown
Using Sentimag
When examining the iron content at the six specified positions along the LN surface (illustrated in Fig. 6 by using Sentimag), we observed a significant variation in iron distribution throughout as illustrated in Fig. 11-B for two individual LNs one with metastases and one without metastases. In this specific case, there is significantly more iron with a larger spread in the LN containing metastasis compared to a LN without metastasis. Similar distribution of iron was evident across all three sensitivity settings of Sentimag, irrespective of the presence of metastasis in the lymph nodes.
It's noteworthy that the iron content determined through the Sentimag substantially overestimates the iron content measured using SPaQ (Fig. 11-C).
Histopathology
During the histopathological evaluation, metastasis was identified in two LNs from two patients (see Table 2). Patient 7 exhibited a macro metastasis exceeding 2 mm, while patient 5 had a micro metastasis of 0.2-2 mm. Additionally, Patient 1 displayed two LNs with isolated tumour cells, each measuring less than 0.2 mm.
Within healthy LNs, iron particles were observed in both the subcapsular space and sinusoids, with macrophages located in their proximity (Fig. 12-AB). Similarly, in the healthy regions within metastatic LNs exhibit a behaviour similar to that of the healthy LNs (Fig. 12-C-E). However, in the metastatic sites, there was a reduced presence of iron pigment, particularly in the areas occupied by tumour cells. Additionally, adipose tissue exhibited lower levels of iron accumulation compared to the healthy LN tissue.
A Perl’s Prussian blue staining with iron deposition in the subcapsular space. B CD68 Macrophage staining with a deposition in the similar subcapsular space. Below; Pathology image of a lymph node with a macro metastasis stained with Perl’s Prussian Blue. C A large iron deposition is found in the healthy glandular tissue (dark pink on the underside). E There is no iron visible in the tumour tissue (light pink at the top of the lymph node)
The comparative ROI to validate the hypothesis that macrophages are involved in the uptake and transport of iron tracer within the node, the staining on CD68 and FE was compared. In the case of both macro and micro metastasis, no iron was observed in the tumour-bearing regions. Additionally, in regions where iron pigment was identified, macrophages were also visible in the CD68 staining (Fig. 13).
Lowfield MRI
Figure 14 illustrates the MRI of the central slice alongside with the pathology images: healthy LN with low (first row) and high (second row) iron content alongside with a metastatic LN with high iron-content (third row). An experienced breast radiologist assessed the MRI scans for tissue type, resolution, and abnormalities.
MRI and corresponding pathology images for a low iron-content LN (first row), a high iron-content LN (second row) and metastatic LN with high iron-content. The MR images are windowed to increase visibility. In the hexagon, a 10 × magnified view shows a fat region in the Perl’s Prussian blue iron staining (without iron pigment). In the square, there is a 10 × magnified view of a large iron deposition in the healthy glandular tissue. In the circular enlargement (10x), it presents the iron staining of tumour tissue (without iron pigment)
As expected, regardless amount of iron trapped and the MRI sequence, there were no significant differences between signal intensities for plastic holder and formalin between the samples. All fat regions, regardless metastatic status, show no signs of iron deposition and no significant difference in formalin normalised MRI. The healthy LN with low iron content shows a large fatty region in the centre of the node without iron uptake, and confirmed iron in the sinusoidal macrophages. The metastatic LN shows subcapsular and parenchymal tumour cells located in the central region, with limited infiltration into the nearby adipose tissue, without presence of local iron enhancement. However, iron deposits were detected within the sinusoids.
Discussion
As 80% of SLNB procedures are deemed clinically unnecessary [2], it imposes avoidable morbidity on these patients. Similarly, to previous recommendations for omitting axillary lymph node dissection in a substantial number of patients [16], responsibly averting unnecessary LN resection through non-invasive preoperative LN staging promises significant individual healthcare advantages. The LowMag protocol allows for the development and initial validation of preoperative SPIO-enhanced MRI staging prior to breast cancer to prevent for unnecessary LN resection.
We observed an average iron content of 0.1% of the injected dose (1 ml SPION = 28 mg Fe) in the SLNs, ranging from 0% to 0.39%. This finding is similar to earlier a pilot studies: i.e. the tracer uptake of 0.36% for a 22.4 mg SPION injection in head and neck cancer [17], and 0.3% for a 1.6 mL (44.6 mg iron) SPION tracer (Resovist) in breast cancer [18].
Earlier studies on visual analysis of axillary LNs in breast cancer patients, using the disparity in MRI signal between before and after SPION injection [19], showed potential in detecting macro metastases. However, visual analysis still presents a potential challenge in terms of consistency among observers. Our previous work [20] and this LowMag protocol attempt to solve this issue by quantifying the MRI of metastatic LN tissue.
The MRI images clearly differentiate between fat, nodal tissue and SPIO tracer (either in signal intensity or in texture), demonstrating a potential of LN imaging using a portable MRI scanner at 0.5 T. However, given the long acquisition time, the scan protocol needs significant time reduction before implementation in clinical setting and pre-operative in vivo scan protocol. Compressed sense reconstruction seems suitable for this purpose [21].
Previous study results of the Lowmag trial showed that patients with fatty SLNs (without metastases) only show SPIO uptake in the cortex. Not in or between fat cells. These SLN showed a thickened cortex after SPIO injection and appeared totally black in the fat-suppressed images. Therefore fat- and water- only images (STIR/DIXON) were used for the distinguish between fatty and metastatic LN.
Although contrast-enhanced MRI showed promise for lymph node staging in earlier studies [22, 23], there are several limitations hindering its use as a replacement for the sentinel node procedure. Firstly, MRI may not always detect all lymph nodes, especially microscopic metastases or nodes deep within the body. Additionally, artefacts or image distortions can complicate interpretation. Furthermore, the cost and availability of MRI technology may limit widespread use, particularly in less developed areas. Moreover, building the expertise and experience necessary for accurate image interpretation may take time. Therefore, the sentinel node procedure remains the preferred standard method for lymph node staging currently. However, research into the application of contrast-enhanced MRI continues, and it may play a more prominent role in this process in the future.
The dispersion of the tracer through the lymphatic system and its uptake in the SLNs is potentially influenced by the injection method (injection site, dose, and timing). Additionally, residual SPIOs at the injection site potentially result in void artifacts in follow-up MRI examinations in near proximity of the injection spot [24]. The low SPIO dose and peritumoural injection used in this study will decrease the detection ratee of the sentinel node during surgery. Compared to a higher dosage of SPIO injected superficially around the nipple, the SLN detection rate is lower. However, we believe that a balance must be struck between effective detection and minimizing side effects. To reduce tattoo formation and MRI artifacts resulting from iron residue during follow-up, we opted for a lower dosage and an iron injection in the resection area. Additionally, for MRI assessment of lymph node metastases, it is important that the iron content in the nodes is not too high, as this could overshadow the entire node. This information on tracer uptake in SLNs can be valuable for dose and protocol optimization.
There are concerns about the future of the sentinel node procedure, since recent and ungoing trial [25] have been investigating the safety of omitting a sentinel lymph node procedure in the case of a negative ultrasound. However, this pertains to a specific group of patients with a small T1 tumour. In our view, there remains a significant patient population that may benefit from a noninvasive lymph node staging, for example patients with larger tumours. Additional, in premenopausal women, the Sentinel Node (SN) status still influences the choice of adjuvant treatment (whether or not to undergo chemotherapy, duration of anti-hormonal therapy, type of chemotherapy for HER2-positive tumours). Furthermore, the study only included breast-conserving treatments (those that were irradiated, including axillary sparing except for partial breast irradiation).
In conclusion, future research efforts may concentrate on enlarging the patient cohort with metastases to enhance our understanding of imaging characteristics and texture analysis, and to establish comparisons with pathology images. Given the challenges associated with expertise and accurate image interpretation, there is a growing interest in leveraging advanced techniques or artificial intelligence algorithms to improve interpretation accuracy. Additionally, addressing the limitations of MRI, particularly in enhancing detection sensitivity for small metastases, will be a key focus area. These endeavors aim to refine the role of MRI in lymph node staging and to facilitate its integration into clinical practice for more effective patient management.
The LowMag protocol holds promise to facilitate development of tools in supporting a comprehensive approach, where axillary surgery is reserved for patients with confirmed metastatic lymph nodes.
Availability of data and materials
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request. Data are located in controlled access data storage at the University of Twente.
References
Kim MK, Park HS, Kim JY, Kim S, Nam S, Park S, Kim SI. The clinical implication of the number of lymph nodes harvested during sentinel lymph node biopsy and its effects on survival outcome in patients with node-negative breast cancer. Am J Surg. 2017;214(4):726–32.
Giammarile F, Vidal-Sicart S, Paez D, Pellet O, Enrique EL, Mikhail-Lette M, Morozova O, Maria Camila NM, Diana Ivonne RS, Delgado Bolton RC, et al. Sentinel Lymph Node Methods in Breast Cancer. Semin Nucl Med. 2022;52(5):551–60.
Douek M, Klaase J, Monypenny I, Kothari A, Zechmeister K, Brown D, Wyld L, Drew P, Garmo H, Agbaje O, et al. Sentinel node biopsy using a magnetic tracer versus standard technique: the SentiMAG Multicentre Trial. Ann Surg Oncol. 2014;21(4):1237–45.
Karakatsanis A, Christiansen PM, Fischer L, Hedin C, Pistioli L, Sund M, Rasmussen NR, Jornsgard H, Tegnelius D, Eriksson S, et al. The Nordic SentiMag trial: a comparison of super paramagnetic iron oxide (SPIO) nanoparticles versus Tc(99) and patent blue in the detection of sentinel node (SN) in patients with breast cancer and a meta-analysis of earlier studies. Breast Cancer Res Treat. 2016;157(2):281–94.
Donker M, van Tienhoven G, Straver ME, Meijnen P, van de Velde CJ, Mansel RE, Cataliotti L, Westenberg AH, Klinkenbijl JH, Orzalesi L, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981–22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014;15(12):1303–10.
Harisinghani MG, Barentsz J, Hahn PF, Deserno WM, Tabatabaei S, van de Kaa CH, de la Rosette J, Weissleder R. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. N Engl J Med. 2003;348(25):2491–9.
Cheng G, Kurita S, Torigian DA, Alavi A. Current status of sentinel lymph-node biopsy in patients with breast cancer. Eur J Nucl Med Mol Imaging. 2011;38(3):562–75.
Richtlijnendatabase [https://richtlijnendatabase.nl/richtlijn/borstkanker/dcis/swk_biopsie.html]
Christenhusz MvS A, ten Haken B, Alic L, Dassen AE. Preventing Postoperative Susceptibility Artifacts caused by iron remnants: Managing image guided injection site. In: EUSOBI: 28–30 September 2023. Spain; 2023: Valencia; 2023.
Sentimag [https://www.endomag.com/products/sentimag/]
van de Loosdrecht MM, Draack S, Waanders S, Schlief JGL, Krooshoop HJG, Viereck T, Ludwig F, Ten Haken B. A novel characterization technique for superparamagnetic iron oxide nanoparticles: The superparamagnetic quantifier, compared with magnetic particle spectroscopy. Rev Sci Instrum. 2019;90(2):024101.
Waanders MV S, Oderkerk TOB, Krooshoop aBtH HJG. Method and apparatus for measuring an amount of superparamagnetic material in an object. In: Twente Uo, editor. Espacenet. G01N27/72 edn. The Netherlands: Patentscope/University of Twente; 2015. p. 11. https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2014081290.
Molenaar L, Loosdrecht MMHyd, Alic L, Baarlen Jv, Meijerink JJHJ, Haken Bt, Broeders IAMJ, Lips DJ. Quantification of Magnetic Nanoparticles in ex vivo Colorectal Lymph Nodes. Nano LIFE. 2022;12(03):2250006.
Molenaar L. Look-up table based on SPaQ measurements to quantify amount of iron in small samples. In: 4TU.ResearchData. 1 edn. 2021.
Sadaf Salamzadeh AC, Lejla Alic. Look-up table for assessment of iron content in Magtrace® samples based on Sentimag®. In: 4TU.ResearchData. University of Twente, Faculty of Science and Technology, Magnetic Detection & Imaging (MDI) Group; 2023.
van der Noordaa MEM, Vrancken Peeters M, Rutgers EJT. The intraoperative assessment of sentinel nodes - Standards and controversies. Breast. 2017;34(Suppl 1):S64-s69.
Nieuwenhuis ER, Kolenaar B, Hof JJ, van Baarlen J, van Bemmel AJM, Christenhusz A, Scheenen TWJ, Ten Haken B, de Bree R, Alic L. A Comprehensive Grading System for a Magnetic Sentinel Lymph Node Biopsy Procedure in Head and Neck Cancer Patients. Cancers (Basel). 2022;14(3):678.
Sekino M, Kuwahata A, Ookubo T, Shiozawa M, Ohashi K, Kaneko M, Saito I, Inoue Y, Ohsaki H, Takei H, et al. Handheld magnetic probe with permanent magnet and Hall sensor for identifying sentinel lymph nodes in breast cancer patients. Sci Rep. 2018;8(1):1195.
Motomura K, Ishitobi M, Komoike Y, Koyama H, Noguchi A, Sumino H, Kumatani Y, Inaji H, Horinouchi T, Nakanishi K. SPIO-enhanced magnetic resonance imaging for the detection of metastases in sentinel nodes localized by computed tomography lymphography in patients with breast cancer. Ann Surg Oncol. 2011;18(12):3422–9.
A Christenhusz FS, de Vries N, Hofman M, Dassen AE, ten Haken B, Alic L. Potential of MR lymphography for LN staging in breast cancer. In: European Congress of Radiology. 2021.
de Arthur Lange LA, ten Haken Bennie, Frank Simonis FJ. Accelerated imaging of resected lymph nodes at high spatial resolution using a portable low-field MRI scanner. In: ISMRM: 2023. Toronto: International Society for Magnetic Resonance in Medicine (ISMRM); 2023.
Schipper RJ, Smidt ML, van Roozendaal LM, Castro CJ, de Vries B, Heuts EM, Keymeulen KB, Wildberger JE, Lobbes MB, Beets-Tan RG. Noninvasive nodal staging in patients with breast cancer using gadofosveset-enhanced magnetic resonance imaging: a feasibility study. Invest Radiol. 2013;48(3):134–9.
Chayakulkheeree J, Pungrassami D, Prueksadee J. Performance of breast magnetic resonance imaging in axillary nodal staging in newly diagnosed breast cancer patients. Pol J Radiol. 2019;84:e413–8.
Christenhusz A, Pouw JJ, Simonis FFJ, Douek M, Ahmed M, Klaase JM, Dassen AE, Klazen CAH, van der Schaaf MC, Ten Haken B, et al. Breast MRI in patients after breast conserving surgery with sentinel node procedure using a superparamagnetic tracer. Eur Radiol Exp. 2022;6(1):3.
Gentilini OD, Botteri E, Sangalli C, Galimberti V, Porpiglia M, Agresti R, Luini A, Viale G, Cassano E, Peradze N, et al. Sentinel Lymph Node Biopsy vs No Axillary Surgery in Patients With Small Breast Cancer and Negative Results on Ultrasonography of Axillary Lymph Nodes: The SOUND Randomized Clinical Trial. JAMA Oncol. 2023;9(11):1557–64.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
All authors contributed to the development of this study protocol and provided input within their respective specialties (see below). AC (technical physician) acted as the study coordinator and drafted the initial version of the manuscript. AD (surgical oncologist) served as the principal investigator of the study and primary caregiver for the patients. AD oversaw the surgical aspect of the study and the manuscript. MS (radiologist) was responsible for the radiology component of the study and the manuscript. MB (pathologist) oversaw the pathology section of the study and the manuscript. SS (university lab manager) was responsible for the measurements and adherence to protocols (Fe counts and MRI sequences). BtH and LA were founders of the study. LA provided the manuscript with an initial revision. All authors reviewed the last version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics Committee (Medical Research Ethics Committees United, MEC-U Nieuwegein, the Netherlands) approval was obtained before the start of inclusion, and all patients provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Christenhusz, A., Dassen, A.E., van der Schaaf, M.C. et al. Magnetic procedure for sentinel lymph node detection and evaluation of metastases: design and rationale of the Lowmag trial. BMC Methods 1, 6 (2024). https://doi.org/10.1186/s44330-024-00006-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s44330-024-00006-3