Abstract
We report the development of a sensitive, fast, and simple method for the detection of prometryn (PRO) in potato and celery using a lateral flow immunochromatographic assay (LFIA). A monoclonal antibody (mAb) against PRO was prepared under optimized conditions, and the 50% inhibition concentration (IC50) for use in ELISA was determined to be 0.6 ng mL−1. Moreover, the cut-off value for use in an LFIA was 50 ng mL−1. To validate the reliability of the developed LFIA strip, real samples were used, and the recovery rate was found to be 99.3–104.3%. The assays were completed within 8 min, demonstrating the suitability of our developed methods for the detection of PRO in potato and celery.
Similar content being viewed by others
Introduction
The aim of using pesticides and herbicides in agriculture is to prevent diseases in crop plants caused by insects, microbes, and to control weeds and other pests. Derivatives of triazine are known to be very toxic and persist for many years in the environment [1, 2]. Prometryn (PRO, Fig. 1a) is a selective internal absorption-conducting triazine herbicide, which is inexpensive and commonly used to control weeds. PRO inhibits the photosynthesis reaction and prevents the growth of pre- and post- emergence broadleaf and grassy weeds in major crops [3,4,5].
PRO is characterized by good water solubility and stable chemical properties, which can directly or indirectly cause pollution of surface water, groundwater, soil, and agricultural products [6,7,8]. For most agricultural products, the Environmental Protection Agency requires that the tolerance of triazine herbicides is 250 ng g−1. The European Union (EU) has established a maximum residue limits (MRLs) for triazine herbicide residues in agricultural products (0.05 mg kg−1) to protect public health [9].
Therefore, a rapid and sensitive method for detecting analytes is essential for on-site monitoring to ensure food safety. Various methods are currently used to detect pesticide residues in agricultural products, including high performance liquid chromatography (HPLC) [10,11,12], gas chromatography (GC) [13, 14], and liquid chromatography tandem mass spectrometry (LC–MS/MS) [15,16,17]. The combination of stir fabric phase sorptive extraction (SFPSE) with LC–MS/MS allows the determination of triazine herbicides in water samples with increased sensitivity, with a method detection limit of 17.1 ng mL−1 for PRO [18]. Under optimal conditions, six types of triazines were extracted using a single-hole hollow molecularly imprinted microspheres (h-MIMs) in cereal samples, followed by HPLC–MS/MS detection with a detection limit of 0.1 ng g−1 for PRO [19].
There is a high demand for rapid, inexpensive, and reliable screening methods to monitor the growing number of pesticides in agricultural, biological, and environmental samples. Although these methods are highly sensitive and reliable, they are relatively complex, time-consuming, labor-intensive, and expensive. Immunoassays are characterized by specificity, high throughput, and sensitivity, as well as being inexpensive and easy to operate. Enzyme-linked immunosorbent assay (ELISA) is a method based on the recognition of antigens by antibodies, enabling the detection of a large number of samples without the need for expensive machines for screening [20,21,22,23]. However, the ELISA method still requires an incubation step for the enzyme substrate reaction, making it unsuitable for on-site detection [24,25,26,27].
The lateral flow immunochromatographic assay (LFIA) is an increasingly popular method for the detection of drug residues because it has the advantages of rapid detection (results can be obtained within 5-10 minutes), long shelf life (12-24 months), easy to use, high sensitivity, specificity, and stability, relatively inexpensive, wide market, and high acceptance. Compared to the ELISA method, LFIA is a more portable, faster, and more suitable assay for testing large numbers of samples in the field [26, 28]. To label nanomaterials for LFIA, the easiest, fastest, and conventional method is to use gold nanoparticles (AuNPs), and results can be obtained by comparing the intensity of the color with positive and negative samples by the naked eyes. Recently, to enhance sensitivity, new nanomaterials have been developed for use as labels, including fluorescent nanoparticles such as: fluorescent microspheres (FMs), quantum dots (QDs), upconverting nanoparticles (UNCNPs), and liposomes with fluorescent dyes [29,30,31].
LFIA has become an increasingly popular method to determine analytes because of rapid and sensitive. It is for the first time to develop a rapid and sensitive LFIA for detecting PRO residues in celery and potato.
Results and Discussion
Characterization of PRO-EDC-KLH and PRO-EDC-BSA
The spectrum characterization of the complete PRO antigen is shown in Fig. 1c and d. The PRO hapten has a maximum absorption at 256 nm, and the carrier protein KLH has a maximum absorption at 280 nm. PRO-EDC-KLH has a strong absorption peak at 245 nm. The superposition and offset of the peak stage proved that the coupling of the hapten PRO and KLH was successful. Similarly, the conjugate PRO-EDC-BSA has the characteristic absorption of BSA and PRO, and the peak shape shows an obvious shift and superposition, demonstrating successful coupling of the hapten PRO with BSA.
Assessment of results and characterization of the LFIA
Three New Zealand rabbits were used to develop specific polyclonal antibodies for herbicide atrazine with IC50 of PRO using the ELISA method was 5.7 µg L−1 [32]. Meanwhile, an imprinted electrochemiluminescence sensor was developed to detect PRO in fish and seawater samples with LOD of 0.010 µg kg−1 and 0.050 µg L−1 [33]. In this study, we developed an anti-PRO mAb and determined its specificity by ELISA (Fig. 1e). PRO belongs to the triazine class of herbicides, and its cross-reactivity with other triazine compounds was determined (Table 1). The chemical structures of ametryn and prometon are highly similar to PRO, and the cross-reactivity (CR) with ametryn and prometon was 28.1% and 18.3%, respectively. The structures of terbutryn, semeron, simetryn, terbumeton, secbumeton, atraton, propazine, terbuthylazine, and gesadural also have certain similarities with the structure of PRO, and the CR values were between 5% and 10%. For other triazine compounds, the CR value was low (< 5%).
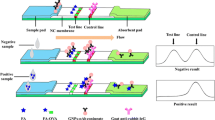
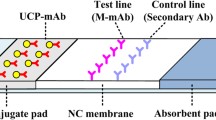
The composition of the LFIA test strip is shown in Fig. 2a. The NC membrane was attached to a PVC bottom plate, and the other pads (the sample pad, conjugate pad, and absorbent pad) were also attached on both ends to overlap the NC membrane. Two lines were created on the NC membrane: the test (T) line, sprayed with PRO-EDC-BSA, and the control (C) line, sprayed with goat anti-mouse antibody.
During the LFIA strip assay, the sample mixture and conjugate (labelled antibody) will interact and flow towards the NC membrane. After several minutes, depends on the concentration of PRO, the red colored bands will appear on both lines. Both C and T lines have colored bands indicate a negative result (no added PRO standard). The absence of a red-colored band at the T line indicates a positive result (see Fig. 2b). The lack of a red-colored band at either the C or T lines indicates that the test procedure is invalid. The color intensity on the C and T lines can be determined using a strip reader (Fig. 2c).
Performance of the LFIA
Several factors that affect the running of the assay need to be considered when performing the LFIA. One of these factors is the addition of the rewetting agents or surfactants, as not every protein is compatible with every surfactant. In this study, we optimized the re-wetting agent by using 5% of PVP, PEG-20000, BSA, and Rhodasurf® ON-870, which were added into the resuspension buffer. The results of optimization using LFLM are shown in Fig. 3a. Based on the stable intensity of the color on the C and T lines, we chose 5% BSA as the surfactant that was added into the resuspension buffer for further experiments.
Optimization strip test of using 4 kinds rewetting agents. A = PVP; B = PEG-2000; C = BSA; and D = Rhodasurf® On-870 (a).—= 0 ng mL−1 and + = 20 ng mL−1. Results after optimization to detect PRO in 0.01 M PBS using LFLM (b), 1–9 represents concentration of 0, 0.25, 0.5, 1, 2.5, 5, 10, 25 and 50 ng mL−1. C = control line. T = test line
To create a range of dilution concentrations, PRO was diluted with 0.01 M PBS: 0.25, 0.5, 1, 2.5, 5, 10, 25 and 50 ng mL−1 (Fig. 3b). The color intensity weakened when a higher concentration of PRO was added and when no color was visible (cut-off) the value was 50 ng mL−1.
Determination of PRO in samples
Samples of potato and celery purchased from a local market in Wuxi, China, which were used to assess the effectiveness of the proposed LFIA. The absence of PRO was evaluated using LC/MS (Fig. S3). Samples were spiked with different concentrations of PRO ranging from 0 to 50 ng mL−1 (Fig. 4a). Deep colored bands will appear on the both lines for each negative sample, with cut-off values of 50 ng mL−1. A calibration curve was constructed by plotting the standard concentrations of PRO against the T/T0 ratio (T = color intensity at the T line with added PRO samples, T0= negative samples) for each concentration (Fig. 4b). The results were obtained within 8 min.
Result after optimization to detect PRO in celery and potato using LFLM strip; (1–9) represents concentration of 0, 0.25, 0.5, 1, 2.5, 5, 10, 25 and 50 ng mL−1 (a). Corresponding quantitative curve of binding rate (T/T0) against concentration of PRO with LFLM strip (b). T/T0 represent ratio of the sample containing PRO sample and sample without PRO
To verify the sensitivity and accuracy of the LFIA based on LFLM, samples were spiked with three different concentrations of PRO (Table 2). Results of the recovery range and CV of the LFIA for detecting of PRO residues in potato and celery were 100.4–104.2%/2.2–5.3% and 99.3–104.3%/2.4–6.7%, respectively.
This study developed a rapid and sensitive assay strip based on fluorescent microspheres. The method detected PRO in less than 10 min, which was faster than using ELISA. Considering both the preparation of the antibody and the sensitivity of the antibody-based AuNPs strip method, the fluorescent microspheres strip was easier to fabricate and had better sensitivity than the antibody-based AuNPs strip method. The application of the fluorescent strips in real samples also demonstrated good detection of the target PRO. These results suggest that the LFIA strip is a fast and sensitive method for detecting PRO residues in potato and celery, making it a promising technique for point-of-care and on-site detection.
Conclusions
A PRO hapten was designed and the complete antigen was synthesized, resulting in the generation of a highly sensitive mAb that can detect PRO in potato and celery using both ELISA and LFLM-based LFIA strip. Through optimization, we were able to achieve an IC50 of 0.6 ng mL−1 for the anti-PRO mAb using the ELISA and found that it also showed cross-reactivity with 11 other triazine herbicides. Additionally, after optimization using LFLM, the cut-off for detecting PRO residues in potato and celery samples using the LFIA strip was 50 ng mL−1. This study presents a reliable, stable, quick, and easy-to-use method for on-site detection of PRO residues in potato and celery.
Materials and Methods
Chemical reagents and instruments
The pesticide standards were PRO, ametryn, prometon, terbutryn, semeron, simetryn, terbumeton, secbumeton, atraton, propazine, terbuthylazine, gesadural, atrazine, cyprazine, simazine, trietazine, and fortrol, all of which were obtained from J&K Scientific Ltd. (Shanghai, China). Gelatin was purchased from Beijing Biodee Biotechnology Co., Ltd. (Beijing, China). All reagents for immunizing the mice and ELISA determination were purchased from Sigma (St. Louis, MO, USA), and for cell experiments were acquired from Nanjing Sunshine Biotech Co., Ltd. (Nanjing, China). ELISA plates were obtained from Wuxi GuoSheng Bio-Engineering Co., Ltd (Wuxi, China). Cell culture plates (96-well) and cell culture flasks were purchased from Wuxi Nest Biological Technology Co., Ltd (Wuxi, China). Fluorescent microspheres (size 200 nm) were obtained from Nanjing Microdetection Bio-Tech Co., Ltd. (Nanjing, China).
The material for LFIA determination were obtained from GoldBio Technology Co., Ltd. (Shanghai, China). Strip cutter machine was obtained from Shanghai Gold Bio-Pharmaceutical Technology Co., Ltd. (Shanghai, China). To determine the color intensity, a strip reader was developed by Determine Bio-Tech Co., Ltd. (Wuxi, China).
Preparation of PRO hapten, protein conjugates, immunization, mAb production, and characterization of ELISA method
Preparation of PRO hapten
The PRO hapten was well designed and can be seen in Fig. 1b. To synthesize it, 10 g cyanuric chloride (54.2 mmol), 24.5 g N,N-diisopropylethylamine (189.8 mmol), and 8.0 g isopropylamine were dissolved in 350 mL of tetrahydrofuran. The mixture was stirred at room temperature for 72 h using a magnetic stirrer, and then water was added to precipitate the product. The resulting solid (8.5 g, product 1) was collected by suction filtration. Next, 2.3 g product 1 (10.0 mmol) was mixed with 60 mL of ethanol, and then slowly added with 2.8 g (20.1 mmol) of potassium hydroxide and 1.5 g 3-mercaptopropionic acid (15 mmol). The reaction mixture was heated and refluxed for 3 h, and then the solvent was removed. The residue was completely dissolved in a 5% NaOH solution, and extracted three times with chloroform (10 mL). The aqueous phase was collected, acidified to pH 4–5 HCl (1 M), and the product was extracted with ethyl acetate. The organic phase was concentrated and crystallized in hexane to obtain 1.1 g of white solid compound 2 (PRO hapten). The chemical synthesis of the PRO hapten was identified by LC–MS in the negative ion mode of the electric spray ion source (Fig. S1).
Procedure of ELISA method
The diluted coating antigen was coated into the 96-well microplates, and incubated at 37 °C for 2 h. After the well was washed three times with washing buffer (0.01 M of PBS containing 0.05% (v/v) Tween-20). The well was blocked for 2 h at 37 °C using blocking buffer 200 µL (0.05 M CBD containing 0.2% (m/v) gelatin), then the wells were washed three times and 50 µL of various concentrations of PRO standard in PBS were added to each well. Then, 50 µL of mAbs in PBS were added to each well, and the plates were incubated at 37 °C for 30 min. The plate was washed three times, and HRP-labeled goat anti-mouse IgG was added to each well followed by incubation at 37 °C for 30 min. Washed the plate for four times, and added 100 µL of 3,3´,5,5´-tetramethylbenzidine (TMB) substrate. After 15 min of reaction at 37 °C in the dark, 50 µL of the stop solution was added to each well and using microplate reader to measure the absorbance at 450 nm.
Preparation of protein conjugation
The PRO hapten contains a carboxyl group, and the immunogen PRO-EDC-KLH and the coating antigen PRO-EDC-BSA were synthesized using the EDC method. First, 2.8 mg PRO hapten, 1.6 mg NHS, and 2.6 mg EDC were weighed and added into a glass bottle. The mixture was dissolved in 400 µL DMF, and allowed to react for 6 h with magnetic stirring to obtain the activation solution (solution 1). Next, BSA and KLH (10 mg) were thoroughly dissolved in CBS (3 mL), and solution 1 was added dropwise. The mixture was reacted with magnetic stirring for 12 h to obtain PRO-EDC-BSA and PRO-EDC-KLH. After preparing a dialysis bag by boiling for 3 min, the immunogen mixture was placed into the dialysis bag and placed in the dialysate (0.01 M PBS) to complete dialysis and remove small molecules that were not coupled to the protein. The immunogen was then stored at −20 °C. A ultraviolet (UV) scanning method was used to characterize the mixture.
Preparation of immunization
All animal studies in this work were performed according to Chinese laws and guidelines which approved by the Animal Ethics Committee of Jiangnan University. Female BALB/c mice, 6–8 weeks old, were immunized with PRO-EDC-KLH. They were first mice immunized using complete Freud’s adjuvant, followed by Freud’s incomplete adjuvant every 3 weeks. We determined the half-maximal inhibitory concentration (IC50) using ELISA, and the highest titer was selected. Next, spleen cells were harvested and fused with Sp 2/0 murine myeloma cells to produce hybridomas. Then resulting hybridomas were injected into the mice to produce ascites. The resulting ascitic fluid was collected, purified, and stored at –20 °C [34].
Characterization of LFIA based on fluorescent-labeled mAbs (LFLM)
To prepare the fluorescent microspheres (Fig. S2), 100 µL of the microspheres was added to borate (BB) buffer (0.05 M, 400 µL), followed by the addition of EDC (30 µL), and the mixture was shaken for 15 min. The mixture was then centrifuged for 15 min at 18,000 × g. After removing the upper layer, BB buffer (0.05 M, 500 µL) was added. The mixture was then added to the anti-PRO mAb (mAb concentration 0.2 ng mL−1, 100 µL), and the mixture was shaken for 2 h at room temperature. After incubation, blocking buffer (55 µL, 10% BSA) was added. After blocking for 2 h, the mixture was centrifuged at 18,000 × g for 15 min. Finally, the residue was collected and 500 µL of resuspension buffer (0.02 M of tris–HCl containing 0.1% Tween-20, 0.1% polyethylene glycol (PEG)-20,000, 1% polyvinylpyrrolidone (PVP), 5% sucrose, 4% trehalose, 2% sorbitol, 1% mannitol, 0.04% NaN3, and 0.2% BSA) was added.
Assembly and evaluation of the test strips
The NC membrane and an absorbent pad were attached to the center of a plastic backing plate. After the control line (C line) was sprayed with goat anti-mouse IgG antibody and test line (T line) was sprayed with PRO-EDC-BSA onto the NC membrane, the test strip was then dried at 37 °C for 24 h.
The conjugated pad was loaded with LFLM diluted 10 folds in resuspension buffer with 5% BSA, then dried for 24 h at 37 °C. After all components (conjugate, sample, and absorbent pad) were attached to the plate, cut the strips into 3 mm for use, 100 µL of the sample solution was dripped onto the sample pad and allowed to react for 8 min. Strip reader was used to detect the color intensity.
The sensitivity of LFIA was determined by diluting the PRO standards in 0.01 M PBS to final concentrations of 0, 0.25, 0.5, 1, 2.5, 5, 10, 25, and 50 ng mL−1. The cut-off value of the LFLM strip was measured by performing a comparison between the negative and positive under UV light in a dark room.
Sample determination
PRO in potato and celery was determined using a simple pre-treatment. Briefly, 1 g samples were weighed into a 10 mL centrifuge tube, added 4 mL of extraction buffer (5% methanol containing 1% Rhodasurf On-870). Vortexed for 5 min. The supernatant was collected and diluted two folds with PBS (0.01 M) containing 1% Rhodasurf On-870 added.
References
Deme P, Azmeera T, Devi BLAP, Jonnalagadda PR, Prasad RBN, Vijaya Sarathi UVR. An improved dispersive solid-phase extraction clean-up method for the gas chromatography-negative chemical ionisation tandem mass spectrometric determination of multiclass pesticide residues in edible oils. Food Chem. 2014;142:144–51.
Hu X, Hu Y, Li G. Development of novel molecularly imprinted solid-phase microextraction fiber and its application for the determination of triazines in complicated samples coupled with high-performance liquid chromatography. J Chromatogr A. 2007;1147(1):1–9. https://doi.org/10.1016/j.chroma.2007.02.037.
Murray A, Lantagne D. Accuracy, precision, usability, and cost of free chlorine residual testing methods. J Water Health. 2015;13(1):79–90. https://doi.org/10.2166/wh.2014.195.
Sun S, Li Y, Lv P, Punamiya P, Sarkar D, Dan Y, et al. Determination of prometryn in vetiver grass and water using gas chromatography-nitrogen chemiluminescence detection. J Chromatogr Sci. 2016;54(2):97–102. https://doi.org/10.1093/chromsci/bmv108.
Wang C, Liu L, Zhang Z, Wu Q, Wang Z. Magnetic biomass activated carbon-based solid-phase extraction coupled with high performance liquid chromatography for the determination of phenylurea herbicides in bottled rose juice and water samples. Food Anal Methods. 2016;9(1):80–7. https://doi.org/10.1007/s12161-015-0181-z.
Xu W, Li J, Feng J, Wang Z, Zhang H. In-syringe temperature-controlled liquid-liquid microextraction based on solidified floating ionic liquid for the simultaneous determination of triazine and phenylurea pesticide in vegetable protein drinks. J Chromatogr B. 2021;1174:122721. https://doi.org/10.1016/j.jchromb.2021.122721.
Zhang LY, Yu RZ, Wang ZB, Li N, Zhang HQ, Yu AM. Determination of triazine herbicides in vegetables by ionic liquid foam floatation solid phase extraction high performance liquid chromatography. J Chromatogr B. 2014;953–954:132–7.
Zhou Q, Xiao J, Ding Y. Sensitive determination of fungicides and prometryn in environmental water samples using multiwalled carbon nanotubes solid-phase extraction cartridge. Anal Chim Acta. 2007;602(2):223–8. https://doi.org/10.1016/j.aca.2007.09.038.
Qin Z, Jiang Y, Piao H, Li J, Tao S, Ma P, et al. MIL-101(Cr)/MWCNTs-functionalized melamine sponges for solid-phase extraction of triazines from corn samples, and their subsequent determination by HPLC-MS/MS. Talanta. 2020;211:120676. https://doi.org/10.1016/j.talanta.2019.120676.
Tian H, Fu H, Xu C, Xu C. Simultaneous determination of three herbicides in honey samples using an aqueous biphasic system coupled with HPLC-MS/MS. Chromatographia. 2019;82(10):1571–7. https://doi.org/10.1007/s10337-019-03781-2.
Tian Y, Liu M, Yang X, Kang C, Wang X. Determination of prometryn residue in color clams by high performance liquid chromatography. Food Ind. 2019;40(12):283–6.
Wang H, Huang X, Qian H, Lu R, Zhang S, Zhou W, et al. Vortex-assisted deep eutectic solvent reversed-phase liquid-liquid microextraction of triazine herbicides in edible vegetable oils. J Chromatogr A. 2019;1589:10–17. https://doi.org/10.1016/j.chroma.2018.12.049.
Gui Y, Ge X, Sun C, Ding Y. Determination and matrix effects of prometryn in fishery products by GC-MS. Fish Sci. 2020;39(6):935–40.
Zhou Q, Lin H, Fu XT, Zhou Y. Determination of prometryn residues in Pyropia spp. by solid phase extraction-gas chromatography-tandem mass spectrometry. J Food Safety Qual. 2018;9(8):1823–8.
Jiang Y, Qin Z, Liang F, Li J, Sun Y, Wang X, et al. Vortex-assisted solid-phase extraction based on metal-organic framework/chitosan-functionalized hydrophilic sponge column for determination of triazine herbicides in environmental water by liquid chromatography-tandem mass spectrometry. J Chromatograp A. 2021;1638:461887. https://doi.org/10.1016/j.chroma.2021.461887.
Li L, Dong WF, Liu MY, Chen X, Liu MY. Determination of prometryne residue in shellfish by ASE-GPC-LC/MSMS. China Food Additives. 2017;7:188–191.
Wu Y, Xu Y, Wang X, Xu Z, Mei Y. Determination of six triazine herbicides in water by solid phase extraction coupled with liquid chromatography tandem mass spectrometry. Chin J Environment Occupation Med. 2021;38(6):654–9.
Roldan-Pijuan M, Lucena R, Cardenas S, Valcarcel M, Kabir A, Furton KG. Stir fabric phase sorptive extraction for the determination of triazine herbicides in environmental waters by liquid chromatography. J Chromatogr A. 2015;1376:35–45. https://doi.org/10.1016/j.chroma.2014.12.027.
Zhao Q, Li H, Xu Y, Zhang F, Zhao J, Wang L, et al. Determination triazine pesticides in cereal samples based on single-hole hollow molecularly imprinted microspheres. J Chromatogr A. 2015;1376:26–34. https://doi.org/10.1016/j.chroma.2014.12.021.
Guo X, Lin L, Song S, Wu A, Liu L, Kuang H, et al. Development of enzyme linked immunosorbent assay and lateral flow immunoassay for the rapid detection of dapsone in milk. New J Chem. 2021;45(40):19097–104. https://doi.org/10.1039/d1nj03247j.
Lu Q, Xu X, Song S, Wu A, Liu L, Kuang H, et al. Development of an immunochromatographic strip for the rapid and ultrasensitive detection of gamithromycin. Food Anal Methods. 2022;15(3):598–606. https://doi.org/10.1007/s12161-021-02146-6.
Pan X, Xu X, Song S, Xu L, Kuang H, Wu X, et al. An ic-ELISA and immunochromatographic strip assay for the detection of 2,4-dichlorophenoxyacetic acid in bean sprouts and cabbage. J Pharm Biomed Anal. 2022;209:114524. https://doi.org/10.1016/j.jpba.2021.114524.
Zeck A, Weller MG, Bursill D, Niessner R. Generic microcystin immunoassay based on monoclonal antibodies against Adda. Analyst. 2001;126(11):2002–7. https://doi.org/10.1039/b105064h.
Chao M, Xu X, Wu A, Song S, Kuang H, Xu C, et al. Gold immunochromatographic strip assay for the detection of triamcinolone acetonide and budesonide in milk. Food Addit Contam. 2022;39(9):1531–43. https://doi.org/10.1080/19440049.2022.2099984.
Li X, Liu L, Guo L, Xu L, Kuang H, Xu C. Development of a colloidal gold immunochromatographic strip for rapid and sensitive detection of nicotine. J Pharm Biomed Anal. 2022;223:115132. https://doi.org/10.1016/j.jpba.2022.115132.
Liu J, Wu A, Song S, Xu L, Liu L, Xu C, et al. Development of an immunochromatographic assay for the rapid screening of pendimethalin in potato and apple. Microchem J. 2022;182:107862. https://doi.org/10.1016/j.microc.2022.107862.
Liu Y, Xu X, Liu L, Xu L, Kuang H, Xu C. Gold-based lateral-flow strip for the detection of penconazole in watermelon and cucumber samples. Food Qual Safety. 2022;6:fyac007. https://doi.org/10.1093/fqsafe/fyac007.
Xu X, Xu X, Song S, Xu L, Zhu Y, Xu C, et al. Development of a broad-specific immunochromatographic assay for the screening of diclofenac and its analogs in health tea and health beverages. Food Biosci. 2022;50:102018. https://doi.org/10.1016/j.fbio.2022.102018.
Sun Y, Song S, Wu A, Liu L, Kuang H, Xu C. A fluorescent paper biosensor for the rapid and ultrasensitive detection of zearalenone in corn and wheat. Anal Methods. 2021;13(35):3970–7. https://doi.org/10.1039/d1ay01149a.
Suryoprabowo S, Xu X, Kuang H, Liu L, Xu C. Methods for quantifying phenolphthalein in slimming tea. J Mat Chem B. 2021;9(18):3856–62. https://doi.org/10.1039/d1tb00510c.
Zhou S, Xu X, Wang L, Guo L, Liu L, Kuang H. A fluorescence based immunochromatographic sensor for monitoring chlorpheniramine and its comparison with a gold nanoparticle-based lateral-flow strip. Analyst. 2021;146(11):3589–98. https://doi.org/10.1039/d1an00423a.
Gascon J, Oubina A, Ballesteros B, Barcelo D, Camps F, Marco MP, et al. Development of a highly sensitive enzyme-linked immunosorbent assay for atrazine-performance evaluation by flow injection immunoassay. Analytica Chimica Acta. 1997;347(1–2):149-62. https://doi.org/10.1016/s0003-2670(97)00269-9.
Zhang RR, Gan XT, Xu JJ, Pan QF, Liu H, Sun AL, et al. Ultrasensitive electrochemiluminescence sensor based on perovskite quantum dots coated with molecularly imprinted polymer for prometryn determination. Food Chemistry. 2022;370:131353.
Chen Z, Wu X, Xu L, Liu L, Kuang H, Cui G. Development of immunocolloidal strip for rapid detection of pyrimethanil. Food Hydrocoll. 2019;30(1):1239–52. https://doi.org/10.1080/09540105.2019.1677566.
Funding
This work was supported by the National Natural Science Foundation of China (22236002).
Author information
Authors and Affiliations
Contributions
C.X. designed the experiments, W.G. and S.S. wrote the manuscript. W.G., A.W., and SS. performed the experiments. L.G. analysed the data. C.X. and S.S. provided discussion and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All animal experiments were in strict accordance with Chinese laws and guidelines which were approved by the Animal Ethics Committee of Jiangnan University and the number of committee ethics approved was JN.No20211115b2400522[446].
Competing interests
All the authors declare no competing financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Fig. S1.
LC-MS identification of prometryn hapten. Fig. S2. Characterization of fluorescence microspheres using transmission electron microscopy. Fig. S3. LC-MS analysis of (a) PRO standard (1000 ng mL−1); negative sample of potato (b); and celery (c).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gu, W., Suryoprabowo, S., Guo, L. et al. Fluorescence strip sensor for sensitive determination of prometryn in foods. One Health Adv. 1, 10 (2023). https://doi.org/10.1186/s44280-023-00005-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/s44280-023-00005-z