Abstract
Background
Perioperative acute ischemic stroke following lung resection is relatively rare, though a devastating complication. Specifically, patients undergoing left upper lung lobectomy for lung cancer are likely to develop perioperative acute ischemic stroke.
Case presentation
A 67-year-old man underwent thoracoscopic left upper lung lobectomy for lung adenocarcinoma; he experienced sudden-onset left hemiparesis and dysarthria on the morning of the second postoperative day. Angiography revealed occlusion of the bulbs of the right internal and external carotid arteries by a giant thrombus, which could not be removed through endovascular thrombectomy. We deployed a stent at the right carotid bifurcation to foist the giant thrombus, achieving revascularization 4 h after the onset. Treatment response was assessed as good improvement with a modified Rankin scale score of 0, and the patient was discharged home 19 days after symptom onset.
Conclusions
We present a unique case of carotid bulb thromboembolism resulting from a giant thrombus following thoracoscopic left upper lung lobectomy, for which endovascular stenting was effective.
Similar content being viewed by others
Background
Perioperative acute ischemic stroke (AIS) is a relatively rare (approximately 0.6%) but devastating complication of lung resection that results in an adjusted 13-fold increase in the inhospital mortality [1,2,3]. Specifically, patients undergoing left upper lung lobectomy (LUL) for lung cancer are likely to develop perioperative AIS (incidence: 1.5–4.5%) [2, 4]. We herein report a case of internal carotid bulb occlusion by a giant thrombus following LUL that was successfully treated with endovascular stenting.
Case presentation
A 67-year-old man with a 40 pack-year smoking history and a medical history of hyperuricemia and hypertension presented with an abnormal shadow in his left upper lung field (Fig. 1a). Chest computed tomography (CT) revealed a 31-mm solid nodule in the left upper lung lobe (S1+2), which showed increased 18F-fluorodeoxyglucose uptake (Fig. 1b and c). Brain contrast-enhanced CT showed no signs of brain metastasis (Fig. 1d). Thus, surgery was scheduled for suspected lung cancer (cT2aN0M0 disease). The serum carcinoembryonic antigen level was elevated to 9.5 ng/mL.
Preoperative imaging. a Chest radiograph shows an abnormal shadow in the left upper lung field. b Chest computed tomography image reveals a 31-mm solid nodule in the left upper lung lobe (S1+2). c Increased 18F-fluorodeoxyglucose uptake is seen within the nodule in the left upper lung lobe. d Brain contrast-enhanced computed tomography image reveals no sign of brain metastasis
Intraoperative frozen section analysis revealed an adenocarcinoma; thus, the patient underwent LUL with lobe-specific nodal dissection via thoracoscopy. Pulmonary artery branches were stapled and initially divided during the surgery. The left superior pulmonary vein (PV) was divided by an endoscopic linear stapler (Fig. 2a and b). Finally, the left upper bronchus was stapled and divided. The final pathological diagnosis was solid-predominant adenocarcinoma (pT2aN0M0, pStage IB). Intraoperative electrocardiography revealed a normal sinus rhythm throughout the surgery.
Thoracoscopic imaging. a The left superior pulmonary vein (LSPV), which did not form a common trunk with the left inferior pulmonary vein, is divided using an endoscopic linear stapler following division of the left pulmonary artery (LPA) branches and prior to division of the left upper bronchus (LUB). b The stump of the LSPV is seen. LPA, left pulmonary artery; LSPV, left superior pulmonary vein; LUB, left upper bronchus
On the morning of the second postoperative day, the patient suddenly developed left hemiparesis and dysarthria with the following vital signs: blood pressure, 130/69 mmHg; heart rate, 60/min; and respiratory rate, 18/min. Twelve-lead electrocardiography revealed a normal sinus rhythm. The D-dimer level was 0.9 μg/mL (reference: ≤ 1.0 μg/mL). “Code stroke” was activated, and the initial neurological examination revealed National Institute of Health Stroke Scale score of 15. Head CT revealed neither signs of intracranial hemorrhage nor early signs of cerebral infarction (Fig. 3a). Emergency carotid endovascular revascularization was initiated at 2 h 50 min following symptom onset. Contrast-enhanced chest CT was not conducted at this time because we prioritized the revascularization. The patient did not consent to transesophageal echocardiography; thus, transthoracic echocardiography was performed, but no signs of a PV stump thrombus or an intracardiac shunt were noted.
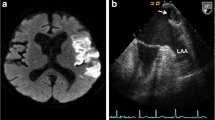
Imaging of the occluded right internal and external carotid arteries treated with endovascular stenting. a Head computed tomography image reveals neither intracranial hemorrhage nor early signs of brain infarction at the onset. b Angiography image shows that the bulb of the right internal carotid artery and the right external carotid artery is occluded by a giant thrombus. c The right internal and external carotid arteries are successfully revascularized by introducing a 10 mm × 40 mm PRECISE stent (Cordis, Miami, FL, USA) at the right carotid bifurcation. d Carotid Doppler ultrasonography image shows that the giant thrombus (TH) remains within the right common carotid artery, protruding from the stent. The blue arrow indicates the recanalized lumen. e Magnetic resonance image obtained on the day after recanalization shows no sign of cerebral infarction. f Magnetic resonance angiography image shows the patency of the vasculature (including the right internal carotid artery). g Carotid Doppler ultrasonography image shows that the thrombus (TH) remains within the right internal carotid artery 10 days after recanalization. h Carotid Doppler ultrasonography image shows that the thrombus has completely disappeared 67 days following recanalization
An angiogram revealed that the bulb of the right internal carotid artery (ICA) and the right external carotid artery was occluded by a giant thrombus (Fig. 3b). We attempted an endovascular forced-suction thrombectomy but failed to remove the giant thrombus. Thus, we decided to deploy a 10 mm × 40 mm PRECISE stent (Cordis, Miami, FL, USA) at the right carotid bifurcation to foist the giant thrombus, which finally lead successful revascularization (Fig. 3c) at 4 h 22 min after the onset. Carotid Doppler ultrasonography demonstrated that the giant thrombus remained in the right common carotid artery, protruding from the stent (Fig. 3d). Immediately following recanalization, the patient’s symptoms improved; the National Institute of Health Stroke Scale score dropped to 1 and 0 at 1 and 3 h after revascularization, respectively. Antithrombotic therapy with clopidogrel (75 mg/day) and aspirin (200 mg/day) was initiated; this was switched to warfarin and clopidogrel (75 mg/day) 7 days after revascularization. The day after revascularization, magnetic resonance imaging revealed no signs of cerebral infarction (Fig. 3e) or carotid artery occlusion (Fig. 3f). Additionally, chest contrast-enhanced CT revealed no signs of PV stump thrombosis. Holter electrocardiography revealed no evidence of atrial fibrillation. Carotid Doppler ultrasonography showed that the thromboemboli still remained within the right ICA 10 days after recanalization (Fig. 3g) but disappeared completely 67 days after recanalization (Fig. 3h). The patient’s condition had improved by a good degree (modified Rankin scale score: 0); the patient was discharged home 19 days after symptom onset. No neurological sequelae were noted for 5 years thereafter.
Discussion
A limitation in our case was that we could not identify the source of thromboembolism. Most cases of perioperative AIS are attributed to large-artery atherosclerosis and cardioembolism; however, one-fourth of the cases remain cryptogenic following standard diagnostic evaluation [5]. Cryptogenic AIS that was deemed nonlacunar and nonatherosclerotic is characterized as an embolic stroke of undetermined source (ESUS) [6], accounting for approximately 17% of all AIS cases [7]. Emerging evidence suggests that a cancer-mediated hypercoagulability may cause ESUS [8,9,10]; currently, reducing cancer activity, including by surgical resection, seems the only way to attenuate the cancer-mediated hypercoagulability and the subsequent risk of AIS development [8].
Based on the current prevailing hypothesis, perioperative AIS following LUL for lung cancer may result from floating thromboemboli that develop due to a turbulent blood flow within a long PV stump [11,12,13,14]. To date, two preventive strategies have been proposed to avoid AIS after a pulmonary lobectomy [15]. The first strategy is the creation of a short PV stump; some surgeons have proposed PV ligation at the level of the pericardial reflection [16, 17]. In light of our case, we started to apply a “dissecting PV last” approach to shorten the PV stump [18]. However, cardiac tamponade may develop if the staple line of the PV involves the pericardial reflection [19, 20]. The second strategy is the early detection of PV stump thrombosis. Contrast-enhanced CT and transesophageal echocardiography are suggested imaging modalities for the detection of PV stump thrombosis; contrast-enhanced CT seems preferrable in post-lobectomy settings for its feasibility [12]. Given that postoperative AIS is likely to develop within 1 week from pulmonary resection [4, 13, 21], routine contrast-enhanced CT is recommended within 1 week postoperatively [15]. If PV stump thrombosis is detected, anticoagulation therapy should be considered in accordance with the management of left atrial thrombosis [22]. In fact, the use of intravenous heparin followed by oral warfarin reportedly leads to the dissipation of PV stump thrombus [23, 24]. Further research is necessary to identify the optimal anticoagulation strategy in balance with the risk of bleeding.
Undoubtedly, appropriate and prompt diagnostic and therapeutic interventions in patients with suspected perioperative AIS are critical for improving their prognosis. “Code stroke,” a rapid response system that prioritizes the hyperacute assessment and management of patients with stroke, is key to optimizing inhospital stroke care [25]. Our hospital introduced a code stroke system in 2010. Our code stroke response team comprises two neurologists and one neuro-interventionist, who are prepared to take action 24/7. According to the 2020 guidelines from the Society for Neuroscience in Anesthesiology and Critical Care, recombinant tissue plasminogen activator is relatively contraindicated following a major surgery within 14 days, while immediate mechanical thrombectomy is recommended for patients with large-vessel occlusion if all selection criteria are met [5]. Thus, endovascular thrombectomy might be a good treatment choice for AIS with large-vessel occlusion following lung resection.
To the best of our knowledge, only 12 cases (including the current case) of endovascular treatment of perioperative AIS following lung resection (Table 1) have been presented to date [26,27,28,29,30,31,32,33]. Our case appears to be the first on endovascular stenting following failed endovascular thrombectomy in a patient who developed perioperative AIS after lung resection. Among the 12 patients, six (50.0%) were finally discharged home, five (41.7%) were transferred to another hospital, and one (8.3%) died of cerebral herniation. Notably, endovascular thrombectomy failed to achieve recanalization in three of the 12 (25.0%) patients. These outcomes are comparable to those reported in a recent meta-analysis on endovascular thrombectomy (mortality, approximately 15%; failure to achieve revascularization, approximately 29%) [34].
In the present case, the thrombus’s size may be the main cause of the failed thrombectomy. Carotid arteries occluded due to giant thrombi are reportedly difficult to revascularize [35, 36]. Diabetes mellitus is a reported independent risk factor for failed mechanical thrombectomy [37]. One study indicated a possible association between the onset-to-groin-puncture time and failed mechanical thrombectomy [37], but another did not [38]. Both atrial fibrillation- and PV stump-associated thrombi are caused due to a turbulent blood flow; however, they majorly differ in terms of the neutrophil and erythrocyte content [39]. In patients with a failed thrombectomy, atrial fibrillation may be associated with recanalization success [38]; however, it remains unclear whether PV stump-based thrombosis are also associated with recanalization success.
Endovascular stenting using self-expandable stents is effective for AIS in the context of carotid circulation [40,41,42,43,44]. Rescue stenting seems to be a valid option for AIS following a failed endovascular thrombectomy [44, 45]. Although rescue stenting requires glycoprotein IIB/IIIa antagonists or oral antiplatelet medications to prevent intra-stent thrombosis, these medications are not associated with symptomatic intracranial hemorrhage [46]. A potential therapeutic alternative for the presented case was catheter crushing and aspiration of the thrombus; however, this could have resulted in distal embolism [36]. Thus, rescue endovascular stenting might be a reasonable therapeutic option for post-lobectomy internal carotid bulb occlusion with a giant thrombus refractory to endovascular forced-suction thrombectomy.
In conclusion, we present a unique case of carotid bulb thromboembolism resulting from a giant thrombus following thoracoscopic LUL, for which endovascular stenting was effective. Further studies should establish an optimal treatment strategy for perioperative AIS secondary to a giant thrombus following lung resection.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the published article.
Abbreviations
- AIS:
-
Acute ischemic stroke
- LUL:
-
Left upper lung lobectomy
- CT:
-
Computed tomography
- PV:
-
Pulmonary vein
- ICA:
-
Internal carotid artery
- ESUS:
-
Embolic stroke of undetermined source
- PVS:
-
Pulmonary vein stenosis
References
Bateman BT, Schumacher HC, Wang S, Shaefi S, Berman MF. Perioperative acute ischemic stroke in noncardiac and nonvascular surgery: incidence, risk factors, and outcomes. Anesthesiol. 2009;110:231–8.
Hattori A, Takamochi K, Kitamura Y, et al. Risk factor analysis of cerebral infarction and clinicopathological characteristics of left upper pulmonary vein stump thrombus after lobectomy. Gen Thorac Cardiovasc Surg. 2019;67:247–53.
Mashour GA, Shanks AM, Kheterpal S. Perioperative stroke and associated mortality after noncardiac, nonneurologic surgery. Anesthesiol. 2011;114:1289–96.
Yamamoto T, Suzuki H, Nagato K, et al. Is left upper lobectomy for lung cancer a risk factor for cerebral infarction? Surg Today. 2016;46:780–4.
Vlisides PE, Moore LE, Whalin MK, Robicsek SA, Gelb AW, Lele AV, Mashour GA. Perioperative care of patients at high risk for stroke during or after non-cardiac, non-neurological surgery: 2020 guidelines from the society for neuroscience in anesthesiology and critical care. J Neurosurg Anesthesiol. 2020;32(3):210–26. https://doi.org/10.1097/ANA.0000000000000686.
Hart RG, Diener HC, Coutts SB, Easton JD, Granger CB, O'Donnell MJ, et al. Cryptogenic Stroke/ESUS International Working Group. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol. 2014;13:429–38.
Hart RG, Catanese L, Perera KS, Ntaios G, Connolly SJ. Embolic stroke of undetermined source: a systematic review and clinical update. Stroke. 2017;48:867–72.
Navi BB, Kasner SE, Elkind MSV, Cushman M, Bang OY, DeAngelis LM. Cancer and embolic stroke of undetermined source. Stroke. 2021;52:1121–30.
Maezono-Kandori K, Ohara T, Fujinami J, Makita N, Tanaka E, Mizuno T. Elevated CA125 is related to stroke due to cancer- associated hypercoagulation. J Stroke Cerebrovasc Dis. 2021;30:106126.
Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021;52:e364–467.
Ohtaka K, Hida Y, Kaga K, et al. Thrombosis in the pulmonary vein stump after left upper lobectomy as a possible cause of cerebral infarction. Ann Thorac Surg. 2013;95:1924–8.
Ohtaka K, Hida Y, Kaga K, et al. Left upper lobectomy can be a risk factor for thrombosis in the pulmonary vein stump. J Cardiothorac Surg. 2014;9:5.
Hattori A, Takamochi K, Shiono S, et al. Multicentre prospective observational study for pulmonary vein stump thrombus after anatomical lung resections. Eur J Cardiothorac Surg. 2021;61:92–9.
Umehara T, Takumi K, Ueda K, et al. Four-dimensional flow magnetic resonance imaging study to explain high prevalence of pulmonary vein stump thrombus after left upper lobectomy. J Thorac Dis. 2020;12:5542–51.
Kimura D, Yamamoto H, Endo S, et al. Postoperative cerebral infarction and arrhythmia after pulmonary lobectomy in Japan: a retrospective analysis of 77,060 cases in a National Clinical Database. Surg Today. 2023;
Nakano T, Kaneda H, Kawaura T, Kitawaki T, Murakawa T. Ligating the pulmonary vein at the pericardial reflection is useful for preventing thrombus formation in the pulmonary vein stump after left upper lobectomy. Gen Thorac Cardiovasc Surg. 2019;67:450–6.
Miyoshi R, Nishikawa S, Tamari S, Noguchi M, Hijiya K, Chihara K. Pulmonary vein thrombosis after lobectomy with vein stump closure by ligation. Asian Cardiovasc Thorac Ann. 2018;26:546–51.
Maru N, Hino H, Utsumi T, et al. Risk factors for postoperative cerebral infarction in lung cancer patients: a retrospective study. J Cardiothorac Surg. 2023;18:132.
Yostumoto T, Nitadori J, Nagayama K, et al. A case of cardiac tamponade following left upper lobectomy for lung cancer. Jpn J Chest Surg. 2015;29:813–7.
Pillai JB, Barnard S. Cardiac tamponade: a rare complication after pulmonary lobectomy. Interact Cardiovasc Thorac Surg. 2003;2:657–9.
Matsumoto K, Sato S, Okumura M, et al. Left upper lobectomy is a risk factor for cerebral infarction after pulmonary resection: a multicentre, retrospective, case-control study in Japan. Surg Today. 2020;50:1383–92.
Hindricks G, Potpara T, Dagres N, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498.
Asai K, Mochizuki T, Iizuka S, Momiki S, Suzuki K. Pulmonary vein stump thrombus: an early complication following upper division segmentectomy of the left lung. Gen Thorac Cardiovasc Surg. 2014;62(4):244–7.
Ichimura H, Ozawa Y, Nishina H, Shiotani S. Thrombus formation in the pulmonary vein stump after left upper lobectomy: a report of four cases. Ann Thorac Cardiovasc Surg. 2013;20(Suppl):613–6.
Nouh A, Amin-Hanjani S, Furie KL, et al. Identifying best practices to improve evaluation and management of in-hospital stroke: a scientific statement from the American Heart Association. Stroke. 2022;53:e165–75.
Ikeda H, Yamana N, Murata Y, Saiki M. Thrombus removal by acute-phase endovascular reperfusion therapy to treat cerebral embolism caused by thrombus in the pulmonary vein stump after left upper pulmonary lobectomy: case report. NMC Case Rep J. 2014;2:26–30.
Sonobe S, Yoshida M, Niizuma K, Tominaga T. Mechanical thrombectomy for acute ischemic stroke arising from thrombus of the left superior pulmonary vein stump after left pneumonectomy: a case report. NMC Case Rep J. 2018;6:17–20.
Kimura D, Fukuda I, Tsushima T, et al. Management of acute ischemic stroke after pulmonary resection: incidence and efficacy of endovascular thrombus aspiration. Gen Thorac Cardiovasc Surg. 2019;67:306–11.
Usui G, Takayama Y, Hashimoto H, et al. Cerebral embolism caused by thrombus in the pulmonary vein stump after left lower lobectomy: a case report and literature review. Intern Med. 2019;58:1349–54.
Masahira N, Ohta T, Tsuno T, et al. Acute proximal anterior circulation occlusion after pulmonary lobectomy treated by endovascular therapy: two case reports. J Neuroendovascular Ther. 2020;14:141–5.
Morinaga Y, Nii K, Sakamoto K, Inoue R, Mitsutake T, Hanada H. Revascularization for in-hospital acute ischemic stroke after video-assisted thoracic surgery: report of 2 cases and literature review. World Neurosurg. 2019;129:28–33.
Kishida N, Fukuda H, Chin M, Handa A, Yamagata S. Pulmonary vein thrombosis as a possible cause of embolic internal carotid artery occlusion under anticoagulation therapy: case report. Jpn J Stroke. 2014;36:275–7.
Fujii Y, Mori Y, Kambara K, Hirota K, Yanada M, Toda S, et al. Pulmonary vein thrombosis and cerebral infarction after video-assisted thoracic surgery of the left upper lobe: a case series. JA Clin Rep. 2020;6(71):2020.
Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723–31.
Yamamoto N, Kuroda K, Yamamoto Y, et al. Long-sheath Introducer-assisted Revascularization (L-SHARE) technique for treating large-vessel occlusion by a giant clot. Intern Med. 2023;15(62):909–13.
Hino T, Sato M, Hayakawa M, et al. A case of acute embolic occlusion of the common carotid artery in which a giant thrombus was retrieved using the parallel stent retriever technique. J Neuroendovascular Ther. 2022;16:87–92.
Lajthia O, Almallouhi E, Ali H, Essibayi et al. Failed mechanical thrombectomy: prevalence, etiology, and predictors. J Neurosurg. 2023 Epub ahead of print.
Heider DM, Simgen A, Wagenpfeil G, Dietrich P, Yilmaz U, Mühl-Benninghaus R, Roumia S, Faßbender K, Reith W, Kettner M. Why we fail: mechanisms and co-factors of unsuccessful thrombectomy in acute ischemic stroke. Neurol Sci. 2020;41(6):1547–55. https://doi.org/10.1007/s10072-020-04244-5.
Hashimoto H, Usui G, Tsugeno Y, et al. Cerebral thromboembolism after lobectomy for lung cancer: pathological diagnosis and mechanism of thrombus formation. Cancers. 2019;11:488.
Iyer SS, White CJ, Hopkins LN, et al. Carotid artery revascularization in high-surgical-risk patients using the Carotid WALLSTENT and FilterWire EX/EZ: 1-year outcomes in the BEACH Pivotal Group. J Am Coll Cardiol. 2008;51:427–34.
Levy EI, Mehta R, Gupta R, et al. Self-expanding stents for recanalization of acute cerebrovascular occlusions. AJNR Am J Neuroradiol. 2007;28(5):816–22.
Zaidat OO, Wolfe T, Hussain SI, et al. Interventional acute ischemic stroke therapy with intracranial self-expanding stent. Stroke. 2008;39:2392–5.
Brekenfeld C, Schroth G, Mattle HP, et al. Stent placement in acute cerebral artery occlusion: use of a self-expandable intracranial stent for acute stroke treatment. Stroke. 2009;40:847–52.
Levy EI, Siddiqui AH, Crumlish A, et al. First Food and Drug Administration-approved prospective trial of primary intracranial stenting for acute stroke: SARIS (stent-assisted recanalization in acute ischemic stroke). Stroke. 2009;40:3552–6.
Chang Y, Kim BM, Bang OY, et al. Rescue stenting for failed mechanical thrombectomy in acute ischemic stroke: a multicenter experience. Stroke. 2018;49:958–64.
Baek JH, Kim BM, Kim DJ, Heo JH, Nam HS, Yoo J. Stenting as a rescue treatment after failure of mechanical thrombectomy for anterior circulation large artery occlusion. Stroke. 2016;47:2360–3.
Acknowledgements
The authors would like to thank Editage (www.editage.jp) for English language editing.
Funding
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
TS, conceptualization, methodology, investigation, and writing—original draft. TK, writing—original draft and supervision. YH, writing—original draft. MI, investigation. NM, writing—review and editing. TU, writing—review and editing. HM, writing—review and editing. YT, writing—review and editing. HH, writing—review and editing. TM, supervision.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Kansai Medical University Hospital Research Ethics Board (file number: H151050), in accordance with the Declaration of Helsinki. Informed consent was obtained from the patient, but the requirement of written consent was waived because this is an anonymized case report.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Saito, T., Kunieda, T., Hashimoto, Y. et al. Internal carotid bulb occlusion by a giant thrombus after thoracoscopic left upper lung lobectomy successfully treated with endovascular stenting: a case report. Gen Thorac Cardiovasc Surg Cases 2, 104 (2023). https://doi.org/10.1186/s44215-023-00116-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s44215-023-00116-4