Abstract
Objectives
To investigate the following: (a) effects of intercostal muscle contraction on sonographic assessment of lung sliding and (b) inter-rater and intra-observer agreement on sonographic detection of lung sliding and lung pulse.
Methods
We used Valsalva and Muller maneuvers as experimental models in which closed glottis and clipped nose prevent air from entering the lungs, despite sustained intercostal muscles contraction. Twenty-one healthy volunteers underwent bilateral lung ultrasound during tidal breathing, apnea, hyperventilation, and Muller and Valsalva maneuvers. The same expert recorded 420 B-mode clips and 420 M-mode images, independently evaluated for the presence or absence of lung sliding and lung pulse by three raters unaware of the respiratory activity corresponding to each imaging.
Results
During Muller and Valsalva maneuvers, lung sliding was certainly recognized in up to 73.0% and up to 68.7% of imaging, respectively, with a slight to fair inter-rater agreement for Muller maneuver and slight to moderate for Valsalva. Lung sliding was unrecognized in up to 42.0% of tidal breathing imaging, and up to 12.5% of hyperventilation imaging, with a slight to fair inter-rater agreement for both. During apnea, interpretation errors for sliding were irrelevant and inter-rater agreement moderate to perfect. Even if intra-observer agreement varied among raters and throughout respiratory patterns, we found it to be higher than inter-rater reliability.
Conclusions
Intercostal muscles contraction produces sonographic artifacts that may simulate lung sliding. Clinical studies are needed to confirm this hypothesis. We found slight to moderate inter-rater agreement and globally moderate to almost perfect intra-observer agreement for lung sliding and lung pulse.
Trial registration
ClinicalTrials.gov registration number.
NCT 02386696.
Similar content being viewed by others
Background
Lung ultrasound (LUS) is more accurate in ruling out a pneumothorax (PNX) than chest X-ray [1,2,3]. Lung sliding, lung pulse, and B-lines are the three sonographic signs proving that the visceral pleura is in contact with the parietal pleura [1,2,3,4]. Lung sliding originates from the movement of the visceral pleura over the parietal pleura during tidal ventilation, being visualized with ultrasound as a sort of “to-and-fro” movement of the pleural line [5,6,7]. Lung pulse reflects changes in heart volume during the cardiac cycle transmitted to the pleural surface [1, 3, 7]. B-lines are vertical artifacts perpendicular to the pleural line, whose semiquantitative assessment reflects water/gas ratio [1, 7]. The presence of lung sliding and lung pulse plays a major role in excluding PNX because, in the absence of interstitial or alveolar lung diseases, B-lines are not commonly seen on the anterior surface of the chest, where partial or occult PNX can be detected in the supine patient. Lung sliding and lung pulse are commonly visualized using the brightness mode (B-mode). The presence of lung sliding can also be confirmed by the seashore sign or excluded by the stratosphere sign using the motion mode (M-mode) [4, 6].
In critical care settings, the specificity of absent lung sliding in ruling out PNX ranges from 78% up to 98%, with a negative predictive value close to 100% [4, 8,9,10]. However, false-positive cases may occur when the visceral pleura is in contact with the parietal pleura, but it does not slide because of the following: (a) no volume of air enters the respiratory system (e.g., apnea or respiratory arrest), one single lung (as in the case of inadvertent bronchial intubation or main bronchus obstruction), and a segment of the lung (e.g., atelectasis); (b) lung or pleural diseases, such as ARDS or pleural adhesions; and (c) dynamic hyperinflation from airway obstruction (e.g., asthma) [4, 11]. The sensitivity of absent lung sliding in ruling out PNX ranges from 81% up to 91% [4, 8,9,10]. A particular category of false-negative cases has been described in recent years. In 2014, Cavaliere and coworkers published a case series of eight postoperative patients in ICU in whom LUS showed artifacts mimicking lung sliding on the side of pneumonectomy, despite the complete absence of the lung and, therefore, of the visceral pleura [12]. The mimicked lung sliding appeared only during spontaneous respiration after successful weaning from mechanical ventilation, and it was not detectable, while the patients were sedated, paralyzed, and on mechanical ventilation. Further cases of spontaneously breathing patients with a confirmed diagnosis of PNX on computed tomography and a simultaneous presence of lung sliding on LUS have been reported [13,14,15]. A possible explanation of this phenomenon might be that chest muscles contraction during spontaneous breathing makes detection of lung sliding difficult. In fact, during inspiration, the parasternal intercostal muscles move ventrally and straighten, while internal intercostal muscles have a prevalent expiratory activity; therefore, the intercostal space width varies through the respiratory cycle [16, 17]. Since the parietal pleura covers the inner surface of the thoracic wall, being separated by intercostal muscles only by the endothoracic fascia, we hypothesized that LUS might detect a false movement of the parietal pleura induced by intercostal muscles contraction occurring in severe dyspnea.
In the light of this evidence, this study was aimed at investigating sonographic effects of the sustained contraction of intercostal muscles. The secondary aim was to assess inter-rater reliability and intra-observer agreement of sonographic detection of lung sliding and lung pulse.
Methods
Design, participants, and settings
This prospective observational study was performed on a cohort of 21 healthy volunteers recruited among physicians, nurses, and other allied healthcare personnel at the Policlinico “A. Gemelli” University Hospital in Rome. Subjects with present or past clinical history of respiratory or cardiovascular diseases were not considered for inclusion.
The study was approved by the Ethics Committee of the Catholic University of the Sacred in Rome: approval number 1436/15. All enrolled subjects gave their informed consent following Ethics Committee recommendations. The study was registered on ClinicalTrials.gov (NCT 02386696).
General protocol
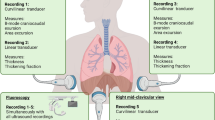
The participants were asked to perform five different respiratory procedures: three respiratory maneuvers (Apnea, Valsalva, and Mueller) and two different respiratory patterns (tidal ventilation during quiet breathing and hyperventilation), as described below. Mueller and Valsalva maneuvers were considered as a physiological model to investigate effects of the sustained contraction of intercostal muscles, since during these conditions no lung volume changes are allowed because of a closed mouth and clipped nose. Before starting the study, all subjects were appropriately trained to perform the required respiratory procedures by one of the authors (D. G. B.).
Ultrasound examination was performed on both sides during each phase by an expert critical care physician proficient in critical care ultrasound (D. G. B.), and all imaging was stored.
Respiratory maneuvers
-
a)
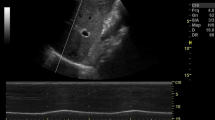
Tidal ventilation at quiet breathing: Initially, the volunteers were asked to breathe quietly to allow the operator to identify the “bat sign” and achieve the correct angle of insonation (Fig. 1A).
-
b)
Apnea: Subjects were asked to hold their breath for 10 s while keeping the intercostal muscles relaxed and inhibiting diaphragmatic activity.
-
c)
Mueller maneuver (Fig. 1B): Subjects were asked to expire forcibly through the mouth, after exhalation of normal tidal volume, to residual volume and to maintain it with a clipped nose. As soon as ultrasound imaging was stabilized, the volunteers performed a sustained maximal inspiratory effort while their mouth closed and nose clipped (Mueller maneuver).
-
d)
Valsalva maneuver (Fig. 1C): Subjects were asked to inspire rapidly close to total lung capacity and maintain this volume actively for 2–3 s; then, they performed a sustained maximal expiratory effort while their mouth closed and nose clipped (Valsalva maneuver).
-
e)
Hyperventilation: Subjects were asked to take deep and rapid breaths to simulate polypnea.
False lung sliding during Muller and Valsalva maneuvers. A Placing the probe perpendicular to two consecutive ribs in the parasternal area allowing visualization of the so-called “bat sign”: the upper and lower ribs are the wings of the bat, and, a little deeper, the pleural line is the body of the bat. B False lung sliding during Muller’s maneuver. M-mode imaging reproducing artifacts mimicking lung sliding which is generated by contractions of parasternal intercostal muscles during Muller’s maneuver. C False lung sliding during Valsalva’s maneuver. M-mode imaging reproducing artifacts mimicking lung sliding which is generated by contractions of parasternal intercostal muscles during the Valsalva maneuver
After each maneuver and before performing the next one, the subjects were allowed to recover by breathing quietly for 2 min at least. The sequence was performed twice on both sides of the chest wall.
Ultrasound assessment and acquisition
LUS was performed while the subjects were seated on a chair with a back support to minimize trunk motion and enable the operator to maintain a steady insonation angle during the imaging acquisition.
The MyLab Five Esaote Ultrasound System (Esaote SpA, Genoa, Italy) was used, equipped with a high frequency 4–13 MHz broadband linear probe, well suited for sonographic examination of pleural line and parasternal intercostal muscles.
Imaging acquisition was obtained by placing the probe longitudinally along the midclavicular line and perpendicular to two consecutive ribs, at the level of the 3rd to the 4th intercostal spaces. This scan offers a good view of the ribs, intercostal muscles, and pleural lines (Fig. 1A). When the correct position of the probe with proper image acquisition was achieved, the operator registered and saved a 10-s video clip in B-mode for each side and two sequences of six consecutive breaths in M-mode during each respiratory maneuver. Videotapes and images were all stored on a memory disc by the principal investigator (D. G. B.).
Ultrasound interpretation and bias assessment
The same researcher (D. G. B.) who performed ultrasound examinations renamed videos and images saved based on a random sequence, making it impossible to trace the corresponding maneuver from the file name. The process was repeated twice so that two groups of files, A and B, were finally obtained, in which the same imaging was stored under different names and sequences.
Three researcher experts in critical care ultrasound (A. C., C. S., F. C.), who were blinded to the file encoding system, examined group A first and group B 15 days later. During each evaluation session, first, each rater was asked to assess whether lung sliding and lung pulse were present in each B-mode video, integrating it with the corresponding M-mode imaging and then without interacting with other investigators. The access to the related M-mode imaging was allowed only after that the three raters had sent the final assessment based on B-mode to the principal investigator (D. G. B.). Then, the assessment based on the integration of B-mode videos and M-mode images was registered and analyzed separately from the assessment based solely on B-mode. Before starting the study, the three examiners agreed on the criteria for lung sliding and lung pulse presence after two meetings.
Statistical analysis
The Shapiro–Wilk test assessed the normal distribution of continuous variables, which is most powerful for small sample sizes with less than 50 patients.
Continuous variables with normal distribution are presented as mean ± standard deviation (SD). Continuous variables with non-normal distribution are presented as the median and interquartile range (IQR). Categorical variables were presented as the number of patients, or percentages, with 95% CIs and were compared using the χ2 test.
Inter-rater and intra-observer agreement was assessed using Cohen’s k test, comparing observed agreement with the expected agreement if the ratings were independent. Cohen’s kappa ranges between − 1 and 1. According to Landis and Koch, values < 0 indicate no agreement, values between 0 and 0.20 slight agreement, values between 0.21 and 0.40 fair agreement, values between 0.41 and 0.60 moderate agreement, values between 0.61 and 0.80 substantial agreement, and values > 0.81 almost perfect agreement [18].
No statistical sample size calculation was performed a priori since this is a physiologic study on healthy volunteers with no previously published trial testing the same hypothesis.
STATA software for Mac (Stata/BE 17 for Mac, StataCorp., 4905 Lakeway College Station, USA) was used. P < 0.05 was considered statistically significant.
Results
Subjects enrolled were all males, aged 38 (± 11) years, and with a body mass index (BMI) of 24.9 (± 2.4) kg/m2. During the study, 420 B-mode clips and M-mode images were recorded, stored, and examined.
The main results are summarized in Tables 1, 2 and 3 and in Supplemental Tables 1 and 2.
Table 1 reports the agreement between what each rater observed and what was expected, across two different respiratory maneuvers and three different respiratory patterns. The error rate in detecting lung sliding and lung pulse has been also reported in Table 1.
Table 2 reports inter-rater agreement of the joint evaluation of B-mode and M-mode imaging for lung sliding and lung pulse.
Table 3 reports the intra-observer reliability of the joint assessment of B-mode and M-mode imaging for lung sliding and lung pulse.
Supplemental tables report inter-rater (eTable 1) and intra-observer (eTable2) agreement for lung sliding and lung pulse, based solely on the evaluation of B-mode imaging. No differences were found between the two hemithoraces.
Tidal breathing
During tidal breathing, the raters failed to recognize lung sliding in up to 42.0% of imaging and lung pulse in up to 29.2% (Table 1). Based on the integration of B-mode and M-mode imaging, inter-rater agreement was slight for lung sliding and slight to moderate for lung pulse (Cohen’s k 0.54 for rater 1 vs 2; Cohen’s k < 0.20 for rater 1 vs 3 and rater 2 vs 3; Table 2). Cohen’s k pointed out perfect or almost perfect intra-observer agreement for raters 2 and 3, for both lung sliding and lung pulse; on the other hand, only moderate intra-observer reliability for lung sliding and slight for lung pulse were found for rater 1 (Table 3). Based on B-mode imaging only, inter-rater agreement was worse for both lung sliding and lung pulse (eTable 1), while intra-observer agreement remained substantially unchanged (eTable 2).
Apnea
During apnea, inter-rater agreement was moderate for lung pulse (Table 2), while inter-rater agreement for lung sliding was moderate to perfect (Cohen’s k 1 for rater 1 vs 3; Cohen’s k 0.41 and 0.46 for rater 2 vs 3 and rater 1 vs 2, respectively; Table 2), based on the integration of B-mode and M-mode. Cohen’s k pointed out a very poor intra-observer agreement for raters 1 and 3 in detecting lung sliding and a perfect intra-observer agreement for rater 2 (Table 3). For lung pulse, a moderate to perfect intra-observer agreement was found for raters 2 and 3 and no agreement for rater 1 (Table 3). Based solely on B-mode imaging, inter-rater agreement was found to be worse while intra-observer agreement to be improved (supplemental tables).
Muller maneuver
During Muller maneuvers, lung sliding was certainly recognized in 14.9 up to 73.0% of imaging and uncertain in up to 56.2% (Table 1). Inter-rater agreement was slight to fair for both lung sliding and lung pulse (Table 2). Cohen’s k pointed out a moderate to almost perfect or perfect intra-observer agreement, for both lung sliding and lung pulse detection, all based on the integration of B-mode and M-mode imaging (Table 3).
Valsalva maneuver
During Valsalva, lung sliding was certainly recognized in up to 68.7% of imaging and uncertain in up to 31.2% (Table 1). Inter-rater agreement was slight for lung sliding and slight to moderate for lung pulse (Table 2). Cohen’s k pointed out a moderate to almost perfect intra-observer agreement for lung sliding and a substantial to perfect intra-observer agreement for lung pulse detection (Table 3).
Hyperventilation
During hyperventilation, lung sliding was erroneously unrecognized in up to 12.5% of imaging and judged uncertain in 27.1 to 35.4% of imaging by two out of three raters (Table 1). All raters failed to correctly recognize lung pulse in most imaging obtained during hyperventilation (Table 1). Inter-rater agreement was slight to fair for lung sliding and very poor for lung pulse (Table 2). Cohen’s k pointed out a wide range of intra-observer agreement among raters, from poor to perfect, both for lung sliding and lung pulse (Table 3).
Based solely on the evaluation of B-mode imaging obtained during Muller and Valsalva maneuvers and hyperventilation trials, inter-rater reliability, as well as intra-observer agreement, remained globally and substantially similar when compared to those based on the integration of B-mode and M-mode imaging, both for lung sliding and lung pulse (supplemental tables).
Discussion
Data from the present study showed that the three raters reported lung sliding in most sonographic imaging obtained during Valsalva and Muller maneuvers, in which glottis and nose closure prevented the air from entering the lungs (Table 1). For this reason, during Valsalva and Muller maneuvers, the visceral pleura does not slide over the parietal pleura, as no air enters the lungs. Therefore, sonographic lung sliding should not be visualized in a healthy subject performing Muller or Valsalva maneuvers. The prolonged and maximal contraction of respiratory muscles performed during the maneuvers may explain these findings. The presence of artifacts mimicking lung sliding and produced by the contraction of parasternal intercostal muscles has been previously described in a case series of eight ICU patients after pneumonectomy. In this case series, artifacts were present during spontaneous breathing but were absent during mechanical ventilation under apneic sedation [12, 14]. Similar artifacts may occur in patients suffering from dyspnea [13,14,15]. Both recently pneumonectomized patients and dyspneic patients may share an increased activity of the parasternal intercostal muscles, whose contraction stabilizes the rib cage and contributes to inspiration by moving against a pleural pressure gradient in opposition to the deflationary action of the diaphragm [16, 17, 19]. Our findings allow to clarify the mechanisms originating these artifacts. In fact, by varying the width of intercostal spaces, the active contraction of intercostal muscles during Muller and Valsalva maneuvers may make the parietal pleura move falsely by dragging. This phenomenon may have significant implications for LUS examination in clinical practice. In fact, in patients recruiting intercostal accessory muscles due to respiratory distress, artifacts mimicking lung sliding (Fig. 1B–C) may erroneously lead clinicians to rule out an eventual PNX.
In our study, artifacts induced by the maximal muscular activity performed during Valsalva and Muller maneuvers, as during hyperventilation trials, may have hidden lung pulse in most videos and images (Fig. 1B–C). Finally, the recognition of the sonographic lung sliding may be insufficient to exclude an eventual PNX if substantial intercostal muscle activation is detected on clinical examination or by ultrasound. However, these findings should be interpreted cautiously since Cohen’s k pointed out only a slight to fair inter-rater agreement for Muller and Valsalva maneuvers.
The secondary endpoint of this study was to assess inter-rater and intra-observer agreement on the sonographic assessment of lung sliding and lung pulse. In this regard, three conditions were studied first. During apnea, the air does not enter the lungs, so we expected to find the lung pulse but not the lung sliding. During tidal breathing and hyperventilation, lung pulse and lung sliding were expected to be present. The three raters correctly identified lung sliding during hyperventilation in most imaging but missed it in almost one-third of cases during tidal breathing. Lung sliding was erroneously recognized in very few imaging obtained during apnea. Furthermore, lung pulse was correctly recognized in almost the total of imaging during apnea and tidal breathing, but it was almost totally missed during hyperventilation. A possible explanation of missing lung pulse during tidal ventilation is that the sign is more or less apparent in the parasternal area depending on the tidal volume and the respiratory pattern, whether predominantly thoracic or abdominal, which may depend on age, gender, and position [17]. On the other hand, lung pulse was probably missed in most hyperventilation imaging because hidden by more profound and more prolonged lung sliding. A remarkable finding was the slight to fair inter-rater agreement for lung sliding detection and slight to moderate for lung pulse, except for apnea condition in which inter-rater agreement resulted much better. In this regard, it is essential to consider that all the raters were proficient in critical care ultrasound, and that their performances were comparable, with more than 5 years of experience each in performing LUS on a daily basis. Thus, a possible explanation of these findings represents, at the same time, a limitation of this study whose design differed significantly from clinical practice. In fact, in the latter, LUS is integrated with a clinical examination so that, for instance, a spontaneously breathing patient can be invited to breathe deeper to magnify lung sliding. Furthermore, the operator who performs LUS examination can achieve unlimited imaging by changing the probe position and insonation angle in case of doubt and comparing B-mode and M-mode in each position. Conversely, in this study, the raters judged based on post-processed clips and images. However, to the best of our knowledge, our findings align with data from a recently published clinical study in which a low agreement was found in the interpretation of LUS for PNX diagnosing on critically ill patients [20].
The main limitation of the present study is represented by the fact that even if Valsalva and Muller’s maneuvers have been carefully performed in the way to minimize pressure changes that could have accounted for recruitment of alveolar units due to volume shifts, it was not possible to completely rule out eventual minimal volume changes that may have been caused by the variable degree of alveolar compression, while subjects maintained the maximal muscular contraction. Therefore, these findings need to be confirmed by larger clinical trials.
However, PNX diagnosis consists in a thoughtful reasoning which requires following a specific algorithm including different ultrasound artifacts to be integrated to the clinical presentation and its evolution [1, 4]. Furthermore, a better understanding of sonographic lung sliding amplitude and their determinants is needed for more accurate diagnosing and monitoring of the critically ill.
Conclusions
This study suggests that the contraction of intercostal muscles may produce sonographic artifacts mimicking lung sliding. However, clinical studies are needed to confirm this finding.
Finally, based on the integrated evaluation of B-mode and M-mode imaging, our study found a slight to moderate inter-rater agreement and a globally moderate to almost perfect intra-observer agreement for lung sliding and lung pulse detection.
Availability of data and materials
No datasets were generated or analysed during the current study.
Abbreviations
- LUS:
-
Lung ultrasound
- PNX:
-
Pneumothorax
- B-mode:
-
Brightness mode
- M-mode:
-
Motion mode
- SD:
-
Standard deviation
- IQR:
-
Inter-quartile range
- BMI:
-
Body mass index
References
Volpicelli G, Elbarbary M, Blaivas M, Lichtenstein DA, Mathis G, Kirkpatrick AW et al (2012) International Liaison Committee on Lung Ultrasound (ILC-LUS) for International Consensus Conference on Lung Ultrasound (ICC-LUS). International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med 38(4):577–91. https://doi.org/10.1007/s00134-012-2513-4
Chan KK, Joo DA, McRae AD, Takwoingi Y, Premji ZA, Lang E, Wakai A (2020) Chest ultrasonography versus supine chest radiography for diagnosis of pneumothorax in trauma patients in the emergency department. Cochrane Database Syst Rev 7(7):CD013031. https://doi.org/10.1002/14651858.CD013031
Soldati G, Testa A, Pignataro G, Portale G, Biasucci DG, Leone A, Silveri NG (2006) The ultrasonographic deep sulcus sign in traumatic pneumothorax. Ultrasound Med Biol 32(8):1157–1163. https://doi.org/10.1016/j.ultrasmedbio.2006.04.006
Volpicelli G (2011) Sonographic diagnosis of pneumothorax. Intensive Care Med 37(2):224–232. https://doi.org/10.1007/s00134-010-2079-y
Rantanen NW (1986) Diseases of the thorax. Vet Clin North Am Equine Pract 2(1):49–66. https://doi.org/10.1016/s0749-0739(17)30732-0
Lichtenstein DA, Menu Y (1995) A bedside ultrasound sign ruling out pneumothorax in the critically ill. Lung sliding Chest 108(5):1345–1348. https://doi.org/10.1378/chest.108.5.1345
Inchingolo R, Zanforlin A, Buonsenso D, Perrone T, Torri E, Limoli G et al (2024) Lung ultrasound signs: the beginning. Part 3-An Accademia di Ecografia Toracica Comprehensive review on ultrasonographic signs and real needs. J Ultrasound Med 43(4):629–641. https://doi.org/10.1002/jum.16397
Lichtenstein DA, Mezière G, Lascols N, Biderman P, Courret JP, Gepner A et al (2005) Ultrasound diagnosis of occult pneumothorax. Crit Care Med 33(6):1231–1238. https://doi.org/10.1097/01.ccm.0000164542.86954.b4
Alrajhi K, Woo MY, Vaillancourt C (2012) Test characteristics of ultrasonography for the detection of pneumothorax: a systematic review and meta-analysis. Chest 141(3):703–708. https://doi.org/10.1378/chest.11-0131
Staub LJ, Biscaro RRM, Kaszubowski E, Maurici R (2018) Chest ultrasonography for the emergency diagnosis of traumatic pneumothorax and haemothorax: a systematic review and meta-analysis. Injury 49(3):457–466. https://doi.org/10.1016/j.injury.2018.01.033
Lichtenstein DA, Lascols N, Prin S, Mezière G (2003) The, “lung pulse”: an early ultrasound sign of complete atelectasis. Intensive Care Med 29(12):2187–2192. https://doi.org/10.1007/s00134-003-1930-9
Cavaliere F, Zamparelli R, Soave MP, Gargaruti R, Scapigliati A, De Paulis S (2014) Ultrasound artifacts mimicking pleural sliding after pneumonectomy. J Clin Anesth 26(2):131–135. https://doi.org/10.1016/j.jclinane.2013.09.011
Ianniello S, Di Giacomo V, Sessa B, Miele V (2014) First-line sonographic diagnosis of pneumothorax in major trauma: accuracy of e-FAST and comparison with multidetector computed tomography. Radiol Med 119(9):674–680. https://doi.org/10.1007/s11547-014-0384-1
Sperandeo M, Maggi M, Catalano D, Trovato G (2014) No sliding, no pneumothorax: thoracic ultrasound is not an all-purpose tool. J Clin Anesth 26(5):425–426. https://doi.org/10.1016/j.jclinane.2014.04.005
Press GM, Miller SK, Hassan IA, Alade KH, Camp E, Junco DD, Holcomb JB (2014) Prospective evaluation of prehospital trauma ultrasound during aeromedical transport. J Emerg Med 47(6):638–645. https://doi.org/10.1016/j.jemermed.2014.07.056
Cala SJ, Kenyon CM, Lee A, Watkin K, Macklem PT, Rochester DF (1998) Respiratory ultrasonography of human parasternal intercostal muscle in vivo. Ultrasound Med Biol 24(3):313–326. https://doi.org/10.1016/s0301-5629(97)00271-8
Taylor A (1960) The contribution of the intercostal muscles to the effort of respiration in man. J Physiol 151(2):390–402. https://doi.org/10.1113/jphysiol.1960.sp006446
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33(1):159–174
Kaneko H, Horie J (2012) Breathing movements of the chest and abdominal wall in healthy subjects. Respir Care 57(9):1442–1451. https://doi.org/10.4187/respcare.01655
Millington SJ, Arntfield RT, Guo RJ, Koenig S, Kory P, Noble V et al (2018) Expert agreement in the interpretation of lung ultrasound studies performed on mechanically ventilated patients. J Ultrasound Med 37(11):2659–2665. https://doi.org/10.1002/jum.14627
Acknowledgements
Not applicable.
Funding
Not applicable, no funding source.
Author information
Authors and Affiliations
Contributions
F.C. and D.G.B. conceived and designed the study. Furthermore, D.G.B. was the expert critical care sonographer who performed all ultrasound exams, and F.C. was one of the three researchers who examined all ultrasound videos and images saved. For this reason, D.G.B. and F.C. gave important contribution to acquisition and interpretation of data. U.M. has revised methodology of the study and made statistical analysis giving substantial contribution to analysis and interpretation of data. A.C. and C.S. were the two other researchers who analyzed ultrasound videos and images giving substantial contribution to acquisition of data and their analysis and interpretation. D.G.B. drafted the paper, whilst F.C. gave important intellectual contribution to the subject revising the final version of the entire paper. M.D. also revised the final version of the manuscript and corrected proofs. L.V. supervised manuscript drafting and revised the final version giving substantial contribution to the interpretation of data. All authors have revised the paper approving it in the final version that has been submitted. All authors agree for all aspects of the work thereby ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethic Committee of the Catholic University of the Sacred Heart in Rome. Ethic Committee approval number: 1436/15. Written informed consent was obtained by each participant to the study.
Consent for publication
Written informed consent for publication was obtained by each participant.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
44158_2024_168_MOESM1_ESM.docx
Supplementary Material 1: Electronic Supplementary Tables. eTable 1. Inter-rater agreement for lung sliding and lung pulse based solely on B-mode imaging. eTable 2. Intra-rater agreement for lung sliding and lung pulse based solely on B-mode imaging.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Biasucci, D.G., Cina, A., Sandroni, C. et al. Influence of intercostal muscles contraction on sonographic evaluation of lung sliding: a physiological study on healthy subjects. J Anesth Analg Crit Care 4, 31 (2024). https://doi.org/10.1186/s44158-024-00168-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s44158-024-00168-0