Abstract
Background
Parasternal intercostal blocks (PSB) have been proposed for postoperative analgesia in patients undergoing median sternotomy. PSB can be achieved using two different approaches, the superficial parasternal intercostal plane block (SPIP) and deep parasternal intercostal plane block (DPIP) respectively.
Methods
We designed the present prospective, observational cohort study to compare the analgesic efficacy of the two approaches. Cardiac surgical patients who underwent full sternotomy from January to September 2022 were enrolled and divided into three groups, according to pain control strategy: morphine, SPIP, and DPIP group. Primary outcomes were was postoperative pain evaluated as absolute value of NRS at 12 h. Secondary outcomes were the NRS at 24 and 48 h, the need for salvage analgesia (both opioids and NSAIDs), incidence of postoperative nausea and vomiting, time to extubation, mechanical ventilation duration, and bowel disfunction.
Results
Ninety-six were enrolled. There was no significant difference in terms of median Numeric Pain Rating Scale at 24 h and at 48 h between the study groups. Total postoperative morphine consumption was 1.00 (0.00–3.00), 2.00 (0.00–5.50), and 15.60 mg (9.60–30.00) in the SPIP, DPIP, and morphine group, respectively (SPIP and DPIP vs morphine: p < 0.001). Metoclopramide consumption was lower in SPIP and DPIP group compared with morphine group (p = 0.01). There was no difference in terms of duration of mechanical ventilation and of bowel activity between the study groups. Two pneumothorax occurred in the DPIP group.
Conclusions
Both SPIP and DPIP seem able to guarantee an effective pain management in the postoperative phase of cardiac surgeries via full median sternotomy while ensuring a reduced consumption of opioids and antiemetic drugs.
Similar content being viewed by others
Introduction
Cardiac surgery techniques have seen rapid advancements over the past two decades; however, full median sternotomy remains the most common approach in cardiac surgery.
To avoid postoperative complications such as prolonged mechanical ventilation, mediastinitis, pulmonary infections [1], and chronic post-sternotomy pain [2, 3], adequate analgesic coverage in the postoperative phase is not only necessary but also recommended to ensure early extubation, mobilization, and discharge from the intensive care unit (ICU).
Unfortunately, the treatment of post-sternotomy pain is often inadequate, as it relies on opioids and other drugs (e.g., cyclo-oxygenase inhibitor, alpha-2 agonist, to provide additive and synergistic analgesic effect) providing minimal benefit to the patient and having significant adverse effects such as nausea, vomiting, ileus, respiratory depression, and sedation.
Therefore, a multimodal based on opioid-sparing analgesia strategies [4] and locoregional anesthesia techniques [5] have been proposed.
Neuraxial anesthesia—mainly thoracic epidural analgesia—and deep plexus blocks have shown to produce excellent analgesia and reduce systemic analgesic requirement [6], but in the cardiac surgery patients, anticoagulant and antiplatelet administration increase the risk of epidural hematoma classically related to neuraxial procedures [7]. Consequently, the American Society of Regional Anesthesia and Pain Medicine is still recommending a conservative approach to neuraxial techniques and other deep blockades, such as thoracic paravertebral block [8].
There is evidence suggesting that relatively new chest wall blocks named parasternal intercostal blocks (PSB) [9] could be effective alternatives for postoperative pain control in patients undergoing median sternotomy [10,11,12,13], even during antiplatelet and anticoagulant therapy [14].
Parasternal intercostal block can be achieved using two different approaches, the superficial parasternal intercostal plane block (SPIP) and deep parasternal intercostal plane block (DPIP), previously known as pectointercostal fascial block (PIFB) and the transversus thoracic plane block (TTPB), respectively [15]. Both techniques are effective in blocking the anterior cutaneous branches of the thoracic intercostal nerves (Th2–6) [16]. To date, in our facility, the use of locoregional anesthesia technique for median sternotomy is not considered a standard, due both to the lack of scientific evidence supporting its widespread use and for the learning curve of the US approach, which is fully mastered only by some clinicians.
Therefore, we designed the present prospective observational cohort study with the aim to compare the efficacy SPIP and DPIP with each other and with the standard treatment on postoperative pain relief in the first 48 postoperative hours after cardiac surgeries performed via medial sternotomy.
Our hypothesis was that PSB techniques would provide at least an equianalgesic effect compared to the standard opioid-based treatment, with less opioid consumption.
Methods
The study has been approved by the local ethics committee (approval number 571/2021, December 25, 2021). All consecutive patients scheduled for cardiac surgeries performed via full median sternotomy between January and September 2022 at two different cardiac surgery centers (Città della Salute e della Scienza Hospital, Turin, Italy, and Azienda Ospedaliera Ordine Mauriziano, Turin, Italy) were included.
Exclusion criteria were age below 18 years, history of opioid abuse, and lack of informed consent. Written informed consent was obtained from all patients included in the study.
Perioperative management
According to local perioperative protocols, general anesthesia was induced with intravenous (IV) midazolam, propofol, or etomidate, plus an IV opiate (Fentanyl or sufentanil, depending on the anesthetist’s choice). Neuromuscular block was achieved with induction bolus followed by continuous infusion of cisatracurium or rocuronium. Anesthesia was maintained by total intravenous infusion of propofol, and intraoperative analgesia was achieved by total intravenous infusion of sufentanil, tailored on patient’s heart rate, blood pressure, and bispectral index values.
Intraoperative care, including cardiopulmonary bypass (CPB), was managed in accordance with standard practice.
At the end of surgery, all patients were transferred to intensive cardiac surgical unit (ICU) for monitoring, respiratory weaning, and standard postoperative management. Sedation was maintained by propofol infusion for as long as deemed necessary. Postoperative pain was managed either with continuous intravenous (IV) morphine infusion or with a multimodal opioid-sparing strategy based on locoregional analgesia (SPIP or DPIP), according to the attending anesthesiologist choice, without interference.
Patients treated with IV morphine infusion received intravenous morphine (about 0.01 mg/kg/h as for standard practice in our center), starting from ICU arrival and titrated to clinical needs until ICU discharge.
In our facility, all the patients receive timed administration of acetaminophen (1 g every 8 h) for 48 h and rescue doses of morphine, tramadol, or ketorolac as needed to ensure pain control.
Both blocks (SPIP and DPIP) were performed at the end of surgery using ropivacaine 3 mg/kg diluted in a total of 60 ml of saline solution (10 ml per point, three points per side) for SPIP and in 40 ml total of saline solution (20 ml per side) for DPIP limiting the maximum dosage of ropivacaine to 300 mg.
Ultrasound-guided superficial parasternal intercostal plane block (SPIP)
This block, originally described by de la Torre [16] in patients undergoing breast surgery, has been recently proposed by Kumar as an effective technique to reduce postoperative pain after sternotomy [10].
With the patient in supine position, after adequate skin disinfection, a linear ultrasound probe was placed on the chest in a parasagittal plane above the 2th, 4th, and 6th intercostal spaces, on the midclavicular line, 2 to 3 cm lateral to the upper third of the sternum. The intercostal spaces have been identified by counting the ribs through the probe.
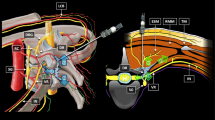
A 22-gauge, 50-mm SonoPlex Stim needle (Pajunk Medical System, Tucker, GA, USA) was advanced via an in-plane approach from the cranial to caudal direction until it reached the interfascial plane between pectoralis major muscle and external intercostal muscle. After the position of the needle tip was confirmed, 10 ml of anesthetic solution was administered (Fig. 1A).
A Ultrasound-guided superficial parasternal intercostal plane block (SPIP). The tip of the needle reaches the interfascial plane between pectoralis major muscle and external intercostal muscle. The dotted area indicates the local anesthetic spread. B Deep parasternal intercostal plane block (DPIP). In the picture clearly visible, the tip of the needle between the interior intercostal muscle and transverse thoracic muscle and the anesthetic solution’s spread
The same procedure was performed in the middle and lower one-third of the sternum and was repeated in the same way on the other side.
Ultrasound-guided deep parasternal intercostal plane block (DPIP)
The DPIP is a deeper fascial plane block originally described by Ueshima [17] for breast cancer resection and used successfully for post-sternotomy pain in both adult [18] and pediatric patients [19].
Patient’s and probe’s position were the same as the SPIP. The maneuver was preceded by an assessment of the position of the internal mammary artery by color Doppler ultrasound to avoid accidental punctures of the vessel.
We used a 22-gauge, 50-mm SonoPlex Stim needle (Pajunk Medical System, Tucker, GA, USA) advanced in an in-plane approach from caudal to cranial direction until the tip of the needle was between the interior intercostal muscle and transverse thoracic muscles. After confirming the position of the needle tip and the correct plane with hydro dissection, 20 ml of anesthetic solution was administered (Fig. 1B).
Data collection and analysis
Demographics characteristics, type and duration of surgery, timing and dosage of pain-related medications, and data regarding postoperative course were collected. Pain was assessed using number rating scale (NRS), and recovery from anesthesia was assessed using Richmond Agitation Sedation Scale (RASS) by attending physicians, who were not blinded to the perioperative analgesic choice. Both scores were collected at 3-h intervals in the first postoperative day and subsequently at least once a day. Recovery was classified “deep to moderate,” when RASS was below −3, or “light to no sedation,” when RASS was between −2 and +1. Nausea was evaluated using a scale from 0 to 3 (0 = absence; 1 = weak nausea; 2 = strong nausea; 3 = very strong nausea) according to local protocol, and bowel function was evaluated using a scale from 0 to 2 (0 = absence of bowel activity; 1 = gas; 2 = feces).
Postoperative morphine use was computed as cumulative dose including any infusion and/or rescue dose. If tramadol was used, we computed tramadol 100 mg equal to morphine 10 mg. The use of nonsteroidal anti-inflammatory drug (NSAID) as rescue therapy was computed separately.
Study outcomes
The primary outcome was postoperative pain evaluated as absolute value of NRS at 12 h. Secondary outcomes were the NRS at 24 and 48, the need for salvage analgesia (both opioids and NSAIDs), incidence of mild adverse effects (i.e., nausea, vomiting, and incorrect catheter placement), quality (RASS), and timing of postoperative course (ICU and hospital length of stay, duration of mechanical ventilation starting from intubation in the operating room, ventilator-free days).
Statistical analysis
According to a previous study [10] in which the median NRS score at 12 h in the control group was 3.5 (range 2.0 to 5.0) and expecting a 50% reduction of pain after SPIP and DPIP with a alpha error of 5% and power 90%, sample size was calculated to be 27 patients in each group.
However, considering potential dropout, a large number of patients was included during the study period.
Data were tested for normal distribution by Shapiro-Wilk test and are expressed as mean and standard deviation (SD) and mean with 95% confidence interval or median with interquartile range 25–75 (IQR), as appropriate. Data analysis was performed for parametric variables with test for independent sample. Kruskal-Wallis nonparametric ANOVA was used for nonparametric continuous variables, followed by Dunn-Bonferroni post hoc test with adjusted significance. Categorical variables were analyzed with Fisher’s exact test, as appropriate. Statistical analyses were performed using SPSS statistics software, version 27 (IBM). A p-value < 0.05 was considered statistically significant.
Results
During the study period, 105 consecutive patients were evaluated for eligibility. Nine patients denied consent and were excluded, while the remaining ninety-six patients were enrolled. After data collection, 32 patients were assigned post hoc to the SPIP group, 32 were assigned to the DPIP group, and 32 to the morphine group. One patient in the DPIP group needed re-intubation due to type 1 respiratory failure 8 h after extubation and was therefore considered dropout and removed from the analysis, leaving 31 patients in the DPIP group (Fig. 2). According with the sample size described above, the enrollment started in January 2022 and was then interrupted in May 2022, having reached an adequate number of patients.
As shown in Table 1, there were no statistical differences between the three groups at baseline.
Total postoperative morphine consumption was 1.0 mg (range 0.0 to 3.0 mg), 2.0 mg (range 0.0 to 5.50 mg), and 15.60 mg (range 9.60 to 30.0 mg) in the SPIP, DPIP group, and morphine group, respectively (SPIP vs morphine: p < 0.001; SPIP vs DPIP: p = 0.47; DPIP vs morphine: p < 0.001) (Table 2). Opioids used postoperatively as rescue doses were equal to 0.0 mg (range 0.0 to 0.0 mg) in the SPIP group and 0.0 mg (range 0.0 to 5.0 mg) in the DPIP group (p = 0.07).
Median NRS were 2.0 (range 0.0 to 3.0), 0.0 (range 0.0 to 4.0), and 2.0 (range 0.0 to 2.25) in the SPIP, DPIP, and morphine group respectively (p = 0.77) at 9 h; 1.0 (range 0.0 to 3.0), 2.0 (range 0.0 to 3.0), and 2.0 (range 1.0 to 2.0) in the SPIP, DPIP, and morphine group respectively (p = 0.98) at 12 h; 1.0 (range 0.0 to 3.0), 1.0 (range 0.0 to 2.0), and 2.0 (range 1.0 to 2.0) in the SPIP, DPIP, and morphine group respectively (p = 0.75) at 24 h; and 0.0 (range 0.0 to 2.0), 1.0 (range 0.0 to 2.0), and 1.0 (range 0.0 to 2.0) in the SPIP, DPIP, and morphine group respectively (p = 0.67) at 48 h.
NSAIDs use as rescue analgesia was not different among groups, while metoclopramide consumption was significantly lower in SPIP and DPIP group compared with morphine group (p = 0.01). There was no significant differences in the incidence of nausea and vomiting at 24 and 48 h and in the time to normal bowel function.
Mechanical ventilation lasted 420.0 min (range 300.0 to 525.0), 363.5 min (range 267.0 to 420.0), and 420.0 min (range 180.0 to 675.0) in the SPIP, DPIP group, and morphine group, respectively (p = 0.25).
Ventilator-free days (median 27—range 27 to 27) and length of ICU stay (median 1 day—range 1 to 1) were comparable between groups (Table 3).
No adverse effects directly attributable to SPIP technique were observed, while two pneumothorax related to the block occurred in the DPIP group. However, these two cases were treated with a conservative approach, leaving the patients spontaneous breathing and were then successfully discharged form ICU. No inhospital death was observed.
Discussion
The main finding of this multicentric, prospective, observational study is that both the superficial parasternal intercostal plane block (SPIP) and deep parasternal intercostal plane block (DPIP) are able to guarantee adequate analgesia with obvious low opioids consumption and a reduction in antiemetics drugs consumption in the 48 h following open cardiac surgeries via full median sternotomy.
SPIP, given the same efficacy in controlling pain, appear safer than DPIP requiring a lower opioid rescue dose. Although both blocks anesthetize the same nerves (the anterior cutaneous branches of the thoracic intercostal nerves [16]), the injection site is completely different: SPIP requires two or three needle punctures on each side, while DPIP requires a single bilateral injection on the 4th/5th intercostal space.
In fact, in the context of parasternal region, the ultrasound imaging reveals a layered structure from the skin to the lung, which includes the following: soft subcutaneous tissues, major pectoralis muscle, exterior intercostal muscles, interior intercostal muscles, internal mammary artery, transversus thoracic muscle, and pleura.
Consequently, while DPIP allows the injection of local anesthetic closest to the anterior branches of the intercostal nerves, the SPIP need two or three injections to ensure an adequate LA spread in the fascial plane [9].
The SPIP, being more superficial, appears to be associated with fewer risks compared with DPIP, since transversus thoracic muscle is located closer to the pleura resulting in a greater risk of pneumothorax [20]. Another possible complication of DPIP is the lesion of the internal mammary artery that courses between the interior intercostal muscle and transverse thoracic muscle [21, 22]; this complication is however easily avoidable using the color Doppler ultrasound.
While there is evidence supporting the efficacy of SPIP and DPIP for the management of acute [11, 23,24,25,26] and chronic [27] post-sternotomy pain, very few studies compared these fascial blocks with other established method of sternotomy pain relief, and only one compared them among themselves [28].
In 2022, Kaya et al. [28] enrolled 39 patients in a double blind comparing the efficacy and safety of DPIP and SPIP. It was found that the two blocks had equal efficacy, in terms of opioid consumption, postoperative NRS, and length of ICU even if, surprisingly, they differed in the time to first rescue dose (280 min in the DPIP group vs 660 min in the SPIP group). Our findings are consistent with these results since the rescue opioid dose was found to be higher in the DPIP group.
It has also been hypothesized that the DPIP requires a greater learning curve compared with SPIP; the transverse thoracic plane is in fact deeper and closer to the pleura, and it could be more difficult to visualize the plane between the internal intercostal muscle and the transverse thoracic muscle. This could result in an increased risk of pneumothorax and unilateral spread of local anesthetic which, in turn, may explain the early and higher rescue opioids demand.
In our study, the postoperative course was similar in the different study groups. In particular, we observed no differences in the duration of mechanical ventilation, length of ICU stays, and/or postanesthesia recovery; this contrasts with the theoretical advantages underlying current opioid-sparing and enhanced recovery protocols [4].
Some elements can explain this lack of statistical significance and limited our findings. First, pain control in morphine group was better than expected and assumed to estimate the sample size. As a result, the study resulted retrospectively underpowered to confirm the observed difference. Secondly, the lack of randomization is a potential source of bias, and, thirdly, all study patients had a particularly short ICU stay justifying, in itself, a better postoperative course [29]. In addition, given the observational study design, PSB were performed by different anesthesiologist, with different skills and experience. Ultimately, failure to standardize intraoperative dosing of sufentanil may have played a role in postanesthesia recovery.
Postoperative nausea and vomiting (PONV) were similar in study groups. This is probably due to the multifactorial genesis of PONV, of which opioids are only one of the triggers. It is also known that in fast-track cardiac surgery, the incidence of PONV is relatively low. Prophylactic administration of antiemetic drugs is therefore usually not necessary [30].
Conclusion
Although the use of traditional opioids is acceptable, both superficial parasternal intercostal plane block (SPIP) and deep parasternal intercostal plane block (DPIP) seem able to guarantee an effective analgesic coverage in the postoperative phase of cardiac surgeries via full median sternotomy while ensuring a reduced consumption of opioids and antiemetic drugs. Future studies are needed to confirm these preliminary results.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- PSB:
-
Parasternal intercostal blocks
- SPIP:
-
Superficial parasternal intercostal plane block
- DPIP:
-
Deep parasternal intercostal plane block
- CPB:
-
Cardiopulmonary bypass
- ASA:
-
American Society of Anesthesiology
- ICU:
-
Intensive care unit
- NRS:
-
Number rating scale
- RASS:
-
Richmond Agitation Sedation Scale
- NSAID:
-
Nonsteroidal anti-inflammatory drug
- PONV:
-
Postoperative nausea and vomiting
References
Agostini P, Cieslik H, Rathinam S et al (2010) Postoperative pulmonary complications following thoracic surgery: are there any modifiable risk factors? Thorax 65:815–818
Kalso E, Mennander S, Tasmuth T et al (2001) Chronic post-sternotomy pain. Acta Anaesthesiol Scand. 45(8):935–939. https://doi.org/10.1034/j.1399-6576.2001.450803.x. (PMID: 11576042)
Meyerson J, Thelin S, Gordh T et al (2001) The incidence of chronic post-sternotomy pain after cardiac surgery--a prospective study. Acta Anaesthesiol Scand 45(8):940–944. https://doi.org/10.1034/j.1399-6576.2001.450804.x. (PMID: 11576043)
Engelman DT, Ben Ali W, Williams JB et al (2019) Guidelines for perioperative care in cardiac surgery: enhanced recovery after surgery society Recommendations. JAMA Surg 154(8):755–766. https://doi.org/10.1001/jamasurg.2019.1153
Capuano P, Toscano A, Rinaldi M et al (2021) Ultrasound-guided thoracic wall nerve blocks in cardiac surgery: is the best yet to come? Minerva Anestesiol 87(3):381–382. https://doi.org/10.23736/S0375-9393.20.15105-8
Svircevic V, Van Dijk D, Nierich A et al (2011) Meta-analysis of thoracic epidural anesthesia versus general anesthesia for cardiac surgery. Anesthesiology 114(2):271–282. https://doi.org/10.1097/ALN.0b013e318201d300
Svircevic V, Nierich AP, Moons KG et al (2011) Thoracic epidural anesthesia for cardiac surgery: a randomized trial. Anesthesiology 114(2):262–270. https://doi.org/10.1097/ALN.0b013e318201d2de
Horlocker TT, Vandermeuelen E, Kopp SL et al (2018) Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine Evidence-Based Guidelines (Fourth Edition). Reg Anesth Pain Med:43. https://doi.org/10.1097/AAP.0000000000000763
Schiavoni L, Nenna A, Cardetta F et al (2022) Parasternal intercostal nerve blocks in patients undergoing cardiac surgery: evidence update and technical considerations. J Cardiothorac Vasc Anesth S1053-0770(22):00542–0. https://doi.org/10.1053/j.jvca.2022.07.025. (Epub ahead of print. PMID: 35995636)
Kumar AK, Chauhan S, Bhoi D et al (2021) Pectointercostal fascial block (PIFB) as a novel technique for postoperative pain management in patients undergoing cardiac surgery. J Cardiothorac Vasc Anesth 35(1):116–122. https://doi.org/10.1053/j.jvca.2020.07.074. (Epub 2020 Jul 30. PMID: 32859487)
Aydin ME, Ahiskalioglu A, Ates I et al (2020) Efficacy of ultrasound-guided transversus thoracic muscle plane block on postoperative opioid consumption after cardiac surgery: a prospective, randomized, double-blind study. J Cardiothorac Vasc Anesth 34(11):2996–3003. https://doi.org/10.1053/j.jvca.2020.06.044. (Epub 2020 Jun 18. PMID: 32665179)
Maeßen T, Korir N, Van de Velde M et al (2023) PROSPECT Working Group of the European Society of Regional Anaesthesia and Pain Therapy. Pain management after cardiac surgery via median sternotomy: a systematic review and procedure-specific postoperative pain management (PROSPECT) recommendation. Eur J Anaesthesiol 20. https://doi.org/10.1097/EJA.0000000000001881 Epub ahead of print. PMID: 37501517
Dost B, De Cassai A, Balzani E et al (2022) Effects of ultrasound-guided regional anesthesia in cardiac surgery: a systematic review and network meta-analysis. BMC Anesthesiol 22(1):409. https://doi.org/10.1186/s12871-022-01952-7. (PMID: 36581838; PMCID: PMC9798577)
Toscano A, Capuano P, Galatà M et al (2022) Safety of ultrasound-guided serratus anterior and erector spinae fascial plane blocks: a retrospective analysis in patients undergoing cardiac surgery while receiving anticoagulant and antiplatelet drugs. J Cardiothorac Vasc Anesth 36(2):483–488. https://doi.org/10.1053/j.jvca.2021.05.037. (Epub 2021 May 24. PMID: 34148801)
El-Boghdadly K, Wolmarans M, Stengel AD et al (2021) Standardizing nomenclature in regional anesthesia: an ASRA-ESRA Delphi consensus study of abdominal wall, paraspinal, and chest wall blocks. Reg Anesth Pain Med 46(7):571–580. https://doi.org/10.1136/rapm-2020-102451
de la Torre PA, García PD, Alvarez SL et al (2014) A novel ultrasound-guided block: a promising alternative for breast analgesia. Aesthet Surg J 34(1):198–200. https://doi.org/10.1177/1090820X13515902. (PMID: 24396082)
Ueshima H, Kitamura A (2015) Blocking of multiple anterior branches of intercostal nerves (Th2-6) using a transversus thoracic muscle plane block. Reg Anesth Pain Med 40:388
Shokri H, Ali I, Kasem AA (2021) Evaluation of the analgesic efficacy of bilateral ultrasound-guided transversus thoracic muscle plane block on post-sternotomy pain: a randomized controlled trial. Local Reg Anesth 12(14):145–152. https://doi.org/10.2147/LRA.S338685. (PMID: 34803399; PMCID: PMC8594901)
Cakmak M, Isik O (2021) Transversus thoracic muscle plane block for analgesia after pediatric cardiac surgery. J Cardiothorac Vasc Anesth 35(1):130–136. https://doi.org/10.1053/j.jvca.2020.07.053. (Epub 2020 Jul 23. PMID: 32798166)
Sepolvere G, Fusco P, Tedesco M et al (2020) Bilateral ultrasound-guided parasternal block for postoperative analgesia in cardiac surgery: could it be the safest strategy? Reg Anesth Pain Med 45:316–317
Sepolvere G, Di Zazzo F, Merola L et al (2021) The correct internal mammary artery anatomy: a topic for ultrasound parasternal block. Saudi J Anaesth 15:233–234
Sepolvere G, Tognu A, Tedesco M et al (2021) Avoiding the internal mammary artery during parasternal blocks: ultrasound identification and technique considerations. J Cardiothorac Vasc Anesth 35:1594–1602
Bloc S, Perot BP, Gibert H et al (2021) Efficacy of parasternal block to decrease intraoperative opioid use in coronary artery bypass surgery via sternotomy: a randomized controlled trial. Reg Anesth Pain Med 46:671–678
Khera T, Murugappan KR, Leibowitz A et al (2021) Ultrasound-guided pectointercostal fascial block for postoperative pain management in cardiac surgery: a prospective, randomized, placebo-controlled trial. J Cardiothorac Vasc Anesth 35:896–903
Zhang Y, Gong H, Zhan B et al (2021) Effects of bilateral pecto-intercostal fascial block for perioperative pain management in patients undergoing open cardiac surgery: a prospective randomized study. BMC Anesthesiol 21:175
Toscano A, Capuano P, Attisani M et al (2022) Transversus thoracic plane block and rectus sheath block for left ventricular assist device implantation via full median sternotomy: a case report. J Card Surg 37(7):2115–2119. https://doi.org/10.1111/jocs.16391. (Epub 2022 Mar 7. PMID: 35254689)
Baki ED, Kavrut Ozturk N, Ayoglu RU et al (2016) Effects of parasternal block on acute and chronic pain in patients undergoing coronary artery surgery. Semin Cardiothorac Vasc Anesth 20:205–212
Kaya C, Dost B, Dokmeci O et al (2022) Comparison of ultrasound-guided pectointercostal fascial block and transversus thoracic muscle plane block for acute poststernotomy pain management after cardiac surgery: a prospective, randomized, double-blind pilot study. J Cardiothorac Vasc Anesth 36:2313–2321
Wong WT, VKW L, Chee YE et al (2016) Fast-track cardiac care for adult cardiac surgical patients. Cochrane Database Syst Rev 12(9):CD003587. https://doi.org/10.1002/14651858.CD003587.pub3
Kogan A, Eidelman LA, Raanani E et al (2003) Nausea and vomiting after fast-track cardiac anaesthesia. Br J Anaesth 91(2):214–217. https://doi.org/10.1093/bja/aeg166
Acknowledgements
None.
Funding
No funding or sponsorship was received for this study or publication of this article.
Author information
Authors and Affiliations
Contributions
AT, PC, and CP designed the study, conducted the study, analyzed the data, and wrote the manuscript. PC performed the statistical analysis. MP, AC, MG, and AO helped design the study and analyze the data. GS and MT helped in review and editing the manuscript. AA, GB, and LB supervised and helped in review and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study has been approved by the local ethics committee (approval number 571/2021, December 25, 2021), and it was in accordance with the Declaration of Helsinki. All patients provided written consent to data collection. The present study was not prospectively registered, but according to the ICMJE definition, registration was not necessary.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Toscano, A., Capuano, P., Perrucci, C. et al. Which ultrasound-guided parasternal intercostal nerve block for post-sternotomy pain? Results from a prospective observational study. J Anesth Analg Crit Care 3, 48 (2023). https://doi.org/10.1186/s44158-023-00134-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s44158-023-00134-2