Abstract
A great attention is subjected to the environmentally friendly natural gas. Compared to other fossil fuels, natural gas is a cleaner burning due to the lower emission of carbon dioxides into the air. Removal of associated hydrocarbons from natural gas streams plays an important role to sell and to achieve the pipeline specification of natural gas. To satisfy the specification of pipelines, the hydrocarbon dew point and water dew point of natural gas must be controlled below the pipeline operating conditions to prevent many problems: two-phase flow and hydrate formation in the system. The main purpose of this paper is to simulate the gas plant process to study the effect of natural gas composition and changing in differential pressure of Joule–Thomson expansion valve on the obtained values of hydrocarbon, water dew points, and cricondentherm temperature.
The results of process simulation using Aspen HYSYS have shown that the control of hydrocarbon dew point, water dew point, and cricondentherm of natural gas is achieved through increasing the Joule-Thomson valve differential pressure. There is an inverse relation between increasing Joule-Thomson valve differential pressure (∆p) and hydrocarbon dew point, water dew point, and cricondentherm to meet the specification of gas pipeline transmission. Increasing differential pressure (∆p) from 14 bar to 24 bar causes a decrease in hydrocarbon dew point, water dew point from −1 to −26°C and from 0 to −18°C, respectively. Cricondentherm is also decreased from 4 to −12°C by increasing differential pressure (∆p) from 14 to 24 bars. The operating conditions at differential pressure below 14 bar is not advisable because cricondentherm temperature does not meet the specification of gas pipeline transmission and hence lead to many problems. Careful adjustment of the operating conditions of gas processing plant is very important by making such simulations to choose the optimum operating conditions which meet gas pipeline transmission.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
The production of natural gas and derivatives play an extremely important role in Egypt. Natural gas consumption is about 53% of the total energy used. Gas total production peak was in 2020 that reached an average rate of 85 BCM/year produced from three areas in Egypt which are Nooros field, West Delta Deep Marine, and Zohr. Currently in 2022, Egypt has three areas of production of natural gas which are Nooros field, West delta deep marine, and Zohr. In 2027, it is expected that gas production will be produced from two areas which are West Nile Delta and Zohr. Natural gas is used in various sectors such as electricity, industry, petroleum, residential, and CNG (compressed natural gas) sectors. The electricity sector is the largest gas consumer, as natural gas consumption reached 62.3% of the total local gas consumption. The industry sector consumes around 22.5% of the total local gas consumption. The petroleum sector consumes about 10.1% of total local consumption. After that, the residential and CNG sectors come at the least sectors that consume natural gas which reached 5.1% of total local natural gas consumption [1,2,3,4].
Natural gas, a highly efficient form of energy, produces a lot of energy and emits fewer pollutants than many other energy sources. It has an increasing demand to provide energy in heating homes, cooking food, and generating electricity. It is considered as a source of hydrocarbon needed in petrochemical feedstocks. It also provides the main ingredients for such varied products as plastics, fertilizers, anti-freezes, and fabrics [5, 6]. Industrial consumers get advantages from operating natural gas combined heat and power (CHP) and combined cooling, heat, and power (CCHP) systems, similar to those used in commercial settings. Natural gas has different compositions depending on the well type and location [5]. Natural gas is formed from methane mainly and smaller amounts of ethane, propane, butane, and heavier hydrocarbons along with varying amounts of water vapors, carbon dioxide, sulfur compounds, and other non-hydrocarbons [7]. Ethane, propane, and butane are known as associated gases or (NGL) [8,9,10,11,12]. They have a variety of different purposes including the improvement of oil recovery in oil wells, providing raw materials for oil refineries or petrochemical plants, and as sources of energy. These NGL components must be recovered to control the dew point of the natural gas stream and also to earn revenue by selling out the separated components. Refrigeration is applied using a direct expansion Joule-Thomson technique or turboexpander to control the dew point of both water and hydrocarbons of natural gas in order to improve gas quality and satisfy the specification of pipelines to obtain saleable and useful energy forms to be used in a wide variety of applications [11,12,13,14]. The hydrocarbon liquid dropout causes some difficulties in gas transmission systems including the increase in pressure drop, reducing in line capacity and some equipment problems [15, 16]. Avoiding liquid dropout, the operating current specifications of gas transmission lines require to be operated above the hydrocarbon dew point or cricondentherm temperature. The achievable control of hydrocarbon dew point and water dew point depends on the differential pressure available and the composition of the feed gas. In industry, there are many widely applied different methods used for hydrocarbon dew point control. Joule-Thomson (J-T) expansion technique is the most preferable method rather than mechanical refrigeration if enough pressure is available [17].
This work aims to simulate the gas processing plant in order to illustrate the optimum operating conditions using Joule–Thomson expansion technique. The effect of feed composition of natural gas and changing in Joule–Thomson valve differential pressure (∆p) on hydrocarbon dew point, water dew point, and cricondentherm temperature is studied to meet the specification of pipeline gas transmission Table 1.
Methods
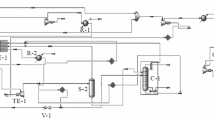
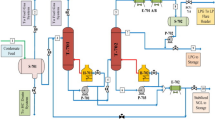
Gases, coming from the wells located in North Nile Delta, contain mainly methane and other hydrocarbons. The feed composition of two wells (C1 and C2) are listed in Table 2. Aspen HYSYS steady-state simulation software version 11 is used to study the effect of different process variables on hydrocarbon dew, water dew point, and cricondentherm in the gas processing plant and choose the optimum process condition. The selected physical property package for the HYSYS model developed is the Peng-Robinson Equation-of-State [18, 19]. The gas processing plant is shown in Fig. 1 and described as follows:
Gas plant block flow diagram [15]
Plant general description
-
Gas streams from the wells are gathered and delivered to the inlet manifold, and methanol solution is injected to prevent hydrate buildup at low temperatures [20].
-
Gases, condensate, and produced water are separated in three-phase separator.
-
Gases are treated in a low temperature separation train which contains gas/gas heat exchanger for cooling, Joule–Thomson (J-T) valve for reducing pressure and a low temperature three-phase separator to separate the condensate liquid, water, and sale gas.
-
Sale gas stream is then recycled to the heat exchanger to cool the feed gas and collected the sale gas as the main product with certain conditions.
-
Condensate is stabilized via a two-stage condensate stabilization unit, stored in tanks, and exported via pumps and pipeline to the national condensate grid.
-
Produced water is degassed and stored in tanks where it is transported by trucks to a safe disposal.
-
The actual plant inlet pressure varies from 51 bars to 66 bars related to the required Joule–Thomson valve differential pressure to ensure high quality of sales gas.
Results and Discussion
The results are divided into four main parts: the first part studies the effect of changing differential pressure of Joule–Thomson expansion valve on the specifications of the natural gas such as hydrocarbon dew point, water dew point, and cricondentherm temperature using composition (C1). The second part illustrates the effect of compositions (C1and C2) of wells on hydrocarbon dew point, water dew point, and cricondentherm. The third part includes the mass balance of LTS train for different feed gas compositions. The fourth part explains the effect of Joule–Thomson valve differential pressure on sale gas gross calorific value using different compositions of natural gas. The conditions of each stream of LTS train are obtained using Aspen HYSYS and the block flow diagram of the gas processing plant is previously shown in Fig. 1.
Effect of Joule–Thomson valve differential pressure (∆p) on the specifications of natural gas using composition (C1)
Effect of (J-T) valve differential pressure (∆p) on hydrocarbon dew point of natural gas
Hydrocarbon dew point plays an important role in the specifications of gas pipeline transmission. Hydrocarbon dew point is the temperature at which the condensation of natural gas occurs when it is cooled at constant pressure [15]. It is necessary to control the hydrocarbon dew point for economical, operational, and safety reasons [20]. The feed of natural gas composition used is from well (1), C1. The effect of differential pressure (∆p) of Joule–Thomson expansion valve on hydrocarbon and water dew points obtained from the process simulation using HYSYS is shown in Fig. 2. There is an inverse relation between increasing in ∆p and the values of hydrocarbon dew point and water dew point. Increasing ∆p from 12 bars to 25 bars causes a decrease in hydrocarbon dew point from −1 to −26.5°C which normally indicates a lower proportion of heavy hydrocarbon components included in this composition of natural gas. This is an important parameter for pipeline transmission specifications. If the natural gas contains a high proportion of heavy hydrocarbons, there is a greater risk of liquid condensate forming in the pipelines. These condensates cause an increase in pressure drop and introduce operational problems resulting from a two-phase formation. These condensates can cause harmful damage such as blockage of pipelines.
Effect of (J-T) valve differential pressure (∆p) on water dew point of natural gas
Water dew point is the temperature at which water vapor begins to condensate. Controlling water dew point of gas prevents the condensation and accumulation of water at low points in the pipelines. The water dew point specification is usually achieved by removing water vapor from the process gas plant stream using different dehydrations or other technologies. Methanol is used as hydrate inhibitor and applied in sufficient quantities. The injected inhibitor absorbs water in the gas to prevent freezing or hydrate formation. The effect of the Joule–Thomson valve differential pressure (∆p) is estimated in Fig. 2. It is clear that increasing the differential pressure obtained from J–T expansion from 14 bars to 24 bars causes a reduction in water dew point from 0 to −18°C which meet the specification of pipeline transmission.
Effect of (J-T) valve differential pressure (∆p) on cricondentherm temperature of natural gas
Cricondentherm can be defined as the maximum temperature at which liquids and vapors can coexist. One phase is present at any pressure at higher temperatures than cricondentherm. The liquid–vapor boundary terminates at a critical point with a critical temperature and critical pressure. By simulating the effect of Joule–Thomson valve differential pressure (∆p) on the cricondentherm temperature as shown in Fig. 2, it is found that increasing differential pressure (∆p) from 14 bars to 24 bars causes a reduction in cricondentherm temperature from 4 to −12°C. This means that operating at values of differential pressure (∆p) lower than 13 bars is not safe because cricondentherm records values greater than 5°C which does not meet gas pipeline transmission.
Effect of different compositions of natural gas on hydrocarbon dew point, water dew point, and cricondentherm
The results of this part illustrate the effect of different compositions of natural gas obtained from wells. Gas compositions mentioned in Table 2 contains well 1 (C1) and well 2 (C2) with various concentrations of different components. The results are given in Figs. 3, 4, and 5. It is obvious that hydrocarbon dew point, water dew point, and cricondentherm are affected by changing the composition of natural gas. A natural gas with a certain composition (C1) listed in Table 2 gives lower values of hydrocarbon dew point and cricondentherm compared to these values obtained using natural gas with composition (C2) which contains more heavier hydrocarbons C6+. This result explained as follows: increasing the concentration of heavier hydrocarbons, especially C6+ causes an increase of hydrocarbon dew point which is very sensitive to the specific components of the gas stream and is strongly influenced by the concentration of the heavier hydrocarbons. The results listed in Table 3 shows that at differential pressure (∆p) equals to 15 bars, the estimated values of hydrocarbon dew point, water dew point, and cricondentherm using composition (C1) are −4.0°C, −1.2°C, and 0.5°C, respectively, whereas those obtained using composition (C2) are −2.0°C, −1.80°C, and 1.0°C, respectively. All these results meet pipelines specification of natural gas transmission.
Mass Balance of LTS Train
Mass balance of each stream of low temperature separation train unit is listed in Table 4 for each composition C1 and C2. The conditions of each stream are listed in Table 5.
Effect of (J–T) valve differential pressure on sale gas gross calorific value using different composition of natural gas, C1 and C2
Gross calorific value of sale gas ranges from 1092 Btu/SCF to 1078.5 Btu/SCF using composition C1 whereas it varies from 1137 Btu/SCF to 1122 Btu/SCF using composition C2 as ∆p increases from 12 bar to 25 bar. These values meet the specification of sale gas listed in Table 1.
Conclusions
The hydrocarbon and water dew points may cause concerns in gas pipelines during transportation. The problem comes from the possibility of liquid condensation in pipelines, leading to issues in metering, pressure drop, and safe operation. It must be controlling hydrocarbon dew point, water dew point, and cricondentherm to meet the specification of pipelines and avoid various problems. Hydrocarbon dew point is a function of gas composition and pressure. The natural gas composition includes the lighter hydrocarbons that reduce the values of hydrocarbon dew point and vice versa. Controlling water dew point can be achieved by elimination of water condensation through pipelines. It is necessary to control the dew points to be below the pipeline operating conditions to prevent two-phase flow and hydrate formation in the system. Natural gas process simulation is very important to get the optimum operating condition that meet the specification of the pipeline as follows: hydrocarbon dew point lower than 5°C at pressure < 70 bars and water dew point equals 0 at 70 bars. Lower values of hydrocarbon dew point, water dew point, and cricondentherm temperature are obtained using raw natural gas composition contains lower content of heavier hydrocarbons whereas the presence of heavier hydrocarbon causes an increase in the hydrocarbon dew point. Also, the optimal operation conditions to meet the specification of pipelines are obtained at differential pressure (∆p) of Joule–Thomson ranging from 14 to 24 bars.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article.
Abbreviations
- BCM:
-
Billion cubic meter unit
- BTU:
-
British thermal unit
- CNG:
-
Compressed natural gas
- CHP:
-
Combined heat and power
- CCHP:
-
Combined cooling, heat, and power
- NGL:
-
Natural gas liquids
- J-T:
-
Joule–Thomson
- LTS:
-
Low temperature separation
- SCF:
-
Standard cubic feet
References
Natural gas - proved reserves, Indexmundi (2020). http://www.indexmundi.com/g/r.aspx?v=98
EGAS Annual Report (2019) https://www.egas.com.eg/annual-reports/2019
EIA, U.S (2021) Energy information administration, natural gas explained. https://www.eia.gov/energyexplained/natural-gas
WORLDOMETERS, Energy (2021) https://www.worldometers.info/energy/egypt- energy.
Abbas N S, Assfour H M, Abdel Wahhab M Z and Ashour E A (2020) About the Egyptian natural gas. An overview, history and prospects. 39: 109 - 116
Xiaomei Z, Fengxia H, Liming Z and Tumeng G (2021) Discussion on water dew point and hydrocarbon dew point of natural gas. 3rd International Conference on Green Energy and Sustainable Development. IOP Conf. Series: Earth and Environmental Science 651. https://doi.org/10.1088/1755-1315/651/3/032090
Elsheemy AA, Ashour FH, Gadalla MA (2018) Maximization of condensate production by revamping of gas-oil separation plant in gulf of Suez. Chem Eng Transact 70:343–348
Ebrahiem EE, Ashour IA, Nassar MM, Abdel Aziz AA (2021) A comparison of natural gas dehydration methods. Yanbu J Eng Sci 15(1):1–16. https://doi.org/10.53370/001c.24332
Sabbagh O, Fanaei MA, Arjomand A (2020) Optimal design of a novel NGL/LNG integrated scheme: economic and exergetic condition. J Thermal Anal Calorimetry 145:5
Gaihuan L, Lin Z, Jinmen H, Huimin L (2022) Technical, economical, and environmental performance assessment of an improved triethylene glycol dehydration process for shale gas. ACS Omega 7(2):1861–1873. https://doi.org/10.1021/acsomega.1c05236
Devold H (2010) Oil and gas production handbook, Edition 2.3. ABB, Oslo
Ahmed OB, Souad A (2021) Energy efficiency improvement of debutanizer column, for NGL separation. Int J Environ Sci Dev 12:255–260
Housam B and Ahmed B (2013) Simulation of the separation of industrially important hydrocarnon mixtures by different distillation techniques using mathematica. Chapter 3:47–78
Xia W, Changjun Li, Yufa He and Wenlong Jia (2018) Operation optimization of natural gas transmission pipelines based on stochastic optimization algorithms: a review. J. Mathematical Problems in Engineering. Article ID 1267045:18. https://doi.org/10.1155/2018/1267045
Noaman A, Ebrahiem E (2021) Comparison of natural gas hydrocarbon dewpointing control methods. J Adv Eng Trend 40:99–116
Poling BE, Prausnitz JM, Connell JP (2001) The properties of gases and liquids, 5th edn
Waele AT (2017) Basics of Joule–Thomson liquefaction and Joule–Thomson cooling. J Low Temp Physics 186:385–403
Peng D Y and Robinson D B (1976) A new two-constant equation of state, industrial and engineering chemistry: fundamentals 15:59-64.
Mondal S K, Uddin M R, Majumder S and Pokhre J (2015) HYSYS simulation of chemical process equipments. Research Gate at: https://www.researchgate.net/publication/281608946
Chatterjee N, Kinard G E and Geist J M(1983) Maximizing production in propane precooled mixed refrigerant LNG plants. Seventh Conference on Liquefied Natural Gas, Jakarta, Indonesia, May 15-19.
Acknowledgements
The authors acknowledge Cairo University, for providing support and for funding the publication of the paper. The authors also acknowledge the Process Department at ENPPI (engineering for petroleum and process industries), for the support provided during the methodology phase.
Funding
Funding was obtained from Cairo University.
Author information
Authors and Affiliations
Contributions
MA wrote up the manuscript and conducted the HYSYS results. NA and AR supervised the HYSYS results, edited, read, and approved the final manuscript. The authors read and approved the final manuscript.
Authors’ information
Prof. Nabil Mahmoud Abdel Monem is currently a professor of Chemical Engineering Department at Faculty of Engineering, Cairo University. Dr. Amr Refay Abdelghany is an associate professor of Chemical Engineering Department at Faculty of Engineering, Cairo University. Eng. Marwa Ahmed El Maghraby is currently a process engineer at ENPPI (Engineering for Petroleum and Process Industries Company).
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
El Maghraby, M.A., El Moniem, N.A. & Abdelghany, A. Controlling hydrocarbon dew point and water dew point of natural gas using Aspen HYSYS. J. Eng. Appl. Sci. 69, 66 (2022). https://doi.org/10.1186/s44147-022-00126-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s44147-022-00126-z