Abstract
Pearl millet (Pennisetum glaucum [L.] R. Br.) is a drought-resilient and nutritious staple food crop widely cultivated in arid and semi-arid regions. Worldwide, pearl millet is ranked the 6th most widely produced cereal crop after wheat, rice, maize, barley, and sorghum, with a total production of 30.5 million tons on 32.1 million hectares. In Burkina Faso, it is the 3rd widely cultivated crop next to sorghum and maize, with a mean yield of 0.8 ton ha−1, far below the potential yield of 3.0 tons ha−1 attributable to various production challenges. Among the production constraints, the parasitic weed Striga species, particularly S. hermonthica is endemic and causes up to 80% yield losses under heavy infestation. Different control methods (e.g., cultural practices, chemicals and bio-herbicides) have been recommended, but they have been largely ineffective due to diverse and complex problems, including the life cycle, seed production, and prolonged seed dormancy of S. hermonthica; poor access and cost of implementation. Breeding for host plant resistance presents a cost-effective, environmentally friendly and affordable method for smallholder farmers to control and reduce Striga infestations and improve pearl millet yields. Therefore, the objectives of this study were to present the impact of S. hermonthica damage on pearl millet production and productivity and assess the effectiveness of different management methods of S. hermonthica with an emphasis on host plant resistance. The first section of the review assesses the impact of Striga infestation on pearl millet production, followed by the developmental stages of Striga, Striga infestation and damage management strategies, breeding for Striga resistance and other Striga control methods. The paper summarises genetic resources, new breeding technologies, and innovations for the precision and speed breeding of Striga-resistant cultivars. The review will guide the use of the best breeding strategies and accelerate the breeding of new pearl millet cultivars that are best-performing and resistant to S. hermonthica to reduce damage incurred by Striga infestations on farmers’ fields in Burkina Faso and related agro-ecologies.
Similar content being viewed by others
Background
Pearl millet (Pennisetum glaucum [L.] R. Br., 2n = 2x = 14) is among the most nutritious and hardy cereal crops in arid and semi-arid regions. In sub-Saharan Africa (SSA), including Burkina Faso, pearl millet is the major cultivated crop after sorghum and maize (INSD 2021; FAOSTAT 2018). Pearl millet is remarkably tolerant to drought, low fertile and saline soils, and higher temperatures, making it the most reliable food supply in dry regions worldwide. Pearl millet is valued for its nutritional profiles and quality food and feed for human wellbeing. For instance, when compared to maize, pearl millet grain has 11–12.5% bio-available protein. The grain comprises higher concentrations of iron, zinc and micro-minerals, including magnesium, calcium, sodium and potassium (Ghatak et al. 2016; Owheruo et al. 2019).
The average productivity of pearl millet is generally low in Africa. In Burkina Faso, a mean grain yield of 0.8 ton ha−1 is reported, comparatively lower than that for maize (1.7 ton ha−1) and sorghum (1.0 ton ha−1) (FAOSTAT 2022). The low productivity of pearl millet is attributable to the combined effect of several production constraints, including lack of improved varieties, S. hermonthica infestation, bird damage, severe drought, and poor soil health (Drabo et al. 2018; Rouamba et al. 2021). Rouamba et al. (2021) reported that S. hermonthica (Del.) Benth was identified to be the major constraint to pearl millet production in five regions of Burkina Faso.
The parasitic weed, Striga species, is the primary constraint to cereal and legume crop production in sub-Saharan Africa (SSA). It is the most noxious weed affecting sorghum, pearl millet, maize and cowpea (Ali et al. 2009). The following Striga species are mainly recognised: S. hermonthica and S. asiatica, which inflict heavy damage to the major cereal crops in SSA. Dafaallah (2019) reported that more than 50 million hectares of agricultural soils under cereal cultivation have been infested by Striga spp. in SSA. The yield losses in cereals due to Striga damage can rise to 80% depending on cultivar susceptibility and the degree of the infestation in SSA (Dafaallah 2019; Kamara et al. 2020). Crop failures and abandonment of cereal production are common in SSA due to high parasitism (Kamara et al. 2020). Most crop damage in Striga-infested fields occurs before Striga emergence, complicating effective parasite control (Dafaallah 2019). During 2017 to 2019, approximately 141 metric tons of cereal grains were produced from 22 million ha in SSA. A yield loss of 1,000 kg ha−1 was reported due to Striga infestation during the same period (Kanampiu et al. 2018). Worldwide, biotic stress (i.e., diseases, pests, and parasitic weeds) is reported to cause about 30% yield loss in cereal crops (Savary et al. 2019). Striga weeds present the most devastating effect on Africa's major cereal and legume crop yield and quality losses. More than 50% of agricultural soils under cereal production is infested by Striga spp. in the region (Rodenburg et al. 2016). An estimated 8.6 million tons annual yield loss is incurred in sorghum and millet in the region (Mallu et al. 2021) for SSA. So far, no Striga-resistant pearl millet cultivars have been bred and deployed in SSA (Jamil et al. 2021; Rouamba et al. 2022). Kountche et al. (2013) identified six and Dayou et al. (2021) one pearl millet with relatively high yields and moderate resistance to Striga.
The management of the Striga epidemics in pearl millet includes cultural practices (e.g., crop rotation, intercropping, optimal soil fertilization, moisture conservation methods, hand-weeding), herbicides, biological control agents (e.g., Fusarium oxysporum f.sp. Strigae [FOS]), resistance breeding and integrated Striga management (Kountche et al. 2013; Jamil et al. 2021; Rouamba et al. 2021; Rouamba et al. 2022). The primary cultural practices are less expensive to employ and helpful in reducing Striga seed bank and infestation. However, they are not widely adopted due to limited access, low farmer acceptance associated with labour shortage, less effective in reducing crop damage and limited access to finance (Murage et al. 2011; Goldwasser and Rodenburg 2013; Mahuku et al. 2017; Franke et al. 2018). According to Hearne (2009), Yoder and Scholes (2010) and Mandumbu et al. (2019), host-resistance is an economical, effective, sustainable approach for managing Striga under smallholder conditions.
Integrating different approaches enables effective Striga management. Combining host plant resistance with a biological control agent (e.g., FOS) effectively reduces Striga counts and emergence (Mrema et al. 2020; Shayanowako et al. 2020; Dossa et al. 2023). The bio-control agent has been successfully used and integrated with resistance breeding in maize (Hassan et al. 2018; Baiyegunhi et al. 2019); Lobulu et al. 2019; Shayanowako et al. 2020; Yacoubou et al. 2021; David et al. 2022) and sorghum (Rebeka et al. 2013; Mrema et al. 2017; Belay 2018; Mrema et al. 2020; Begna 2021). However, this technology is yet to be explored in pearl millet production solo or in combination with other control methods in Burkina Faso and elsewhere. Therefore, the objectives of this study were to present the impact of S. hermonthica damage on pearl millet production and assess the effectiveness of different management methods of S. hermonthica with an emphasis on host plant resistance. The review discusses the impact of Striga infestation on pearl millet production, followed by the developmental stages of Striga, infestation and management strategies, breeding for Striga resistance and other Striga control methods. The paper summarises genetic resources, new breeding technologies, and innovations for developing Striga-resistant cultivars with precision and speed breeding methods.
The impact of Striga on crop production
Striga hermonthica is an obligate and hemi parasitic weed of cereals and legumes. It is a major and continuing threat to crop production in SSA, the Middle East, and Asia (Parker 2012). Striga affects the livelihoods of millions of people in Africa and causes annual yield loss with a monetary value of 7–10 billion US$. Under severe infestation, Striga could lead to entire crop losses (Scholes and Press 2008; Rodenburg et al. 2010; Kountche et al. 2019). The parasite causes significant crop damage, such as stunted plant growth, leaf chlorosis, and reduction of the host's photosynthetic capacity. Due to Striga damage a productivity loss of 80% was reported in cereals, including pearl millet in SSA (Dafaallah 2019). Annual yield losses reaching 8.6 million tons have been reported in sorghum and pearl millet production (Mallu et al. 2021).
Striga hermonthica infestation causes up to 80% yield losses in pearl millet production in Burkina Faso (Rouamba et al. 2021). Wilson et al. (2004) reported grain losses in pearl millet due to Striga ranging between 10 and 95% depending on the susceptibility of the variety, agroecology and cultural practices. The salient features of S. hermonthica damage include stunted plant growth, yellowing and scorching of the leaves, and death of parasitized plants (Sibhatu 2016; Rouamba et al. 2021). A total crop loss with heavy infestations has been reported due to its pervasive nature of the weed (Mbuvi et al. 2017; Menkir et al. 2020). In West Africa, countries including Burkina Faso, Gambia, Mali, Niger, Nigeria, Senegal, and Togo are severely affected by the scourge of Striga (Jamil et al. 2022). Dawud (2018) reported an increasing trend in S. hermonthica occurrence and damage in pearl millet growing areas in Nigeria. Early-generation Striga control reduces yield losses and prevents subsequent spread to previously unaffected areas (Scholes and Press 2008; Kountche et al. 2016).
Developmental stages of Striga
Striga can not survive and grow without the host plant (Cimmino et al. 2018). In 10 weeks after germination, Striga complete its life cycle (Yacoubou et al. 2021). The release of germination stimulants, mainly strigolactones (SLs), by the host plant's roots induces Striga seed germination (Yoneyama et al. 2010; Joel and Bar 2013; Al-Babili and Bouwmeester 2015). After successful germination, the radicle of Striga grows toward the host roots, during which the host perceives and produces chemicals such as 2,6-dimethoxy-1,4-benzoquinone. Host-derived haustorium-inducing factors inhibit the growth of the radicle, followed by cell division and enlargement and root hair proliferation (Goyet et al. 2019).
Striga penetrates the host epidermis by the distal cells of the haustorium (Spallek et al. 2013). The periclinal and anticlinal cells of the haustorium undergo series of cell divisions leading to Striga growth into the cortex of host plants to siphon water and nutrients (Hood et al. 1998; Yoshida et al. 2010). Up to 500,000 Striga seeds are produced per plant. The seeds remain dormant in the soil for 20 years (Lobulu et al. 2019).
Management strategies of Striga infestation and damage
Striga grows in agricultural lands with low soil moisture and fertility associated with cereal monocropping, decreased fallow, and minimal input of organic or inorganic fertilizers (Groote et al. 2005). There are various strategies, solo or in combination recommended to manage Striga. The control measures can be grouped into cultural, chemical, biological, genetic and a combination of these (Mbwika et al. 2011; Sibhatu 2016).
Cultural control method
In Burkina Faso, smallholders routinely use cultural practices to manage Striga (Fig. 1). Cultural practices to control Striga include hand-weeding (Rouamba et al. 2021), cereals and legumes intercropping (Lee and Thierfelder 2017; Mutyambai et al. 2019; Jamil et al. 2021), soil moisture management (Rouamba et al. 2021), mixed cropping and crop rotation (Kuyah et al. 2021), cover cropping (Randrianjafizanaka et al. 2018; Rich 2020), push–pull technology (Niassy et al. 2022), and soil fertilization (Dawud 2017). Cultural control strategies aid in reducing Striga seed proliferation and slow down seed germination and growth (Silberg et al. 2021). Push–pull’ is an approach that involves intercropping fields with a repellent and an attractant trap plant. The push–pull technology was developed to control Striga in resource-poor farming systems by repelling the weed from the major food crops while simultaneously attracting it to a trap crop (Ndayisaba et al. 2020). The method explores an allelopathic effect of the intercrop root exudates in suppressing the germination of Striga seed (Khan et al. 2010). For instance, the root secretes of Desmodium promote Striga seed germination and prevent the attachment of the young plants to host roots through radical growth inhibition. This system results in the depletion of Striga seed bank (Ndayisaba et al. 2020). However, most cultural control strategies are perceived as unaffordable, labour-intensive, or incompatible with other farm operations (Sibhatu 2016) and have thus not been applied widely. Hand weeding is widely practised by smallholder farmers using family labour. However, this method is laborious, time-consuming, and less efficient to reduce Striga seed bank and crop damage (Mahuku et al. 2017). Integrated Striga management (ISM) is the most effective way to control S. hermonthica (Magallon-Servín et al. 2020). Nevertheless, smallholder farmers do not use ISM due to limited access to a combination of resources (David et al. 2022).
Photos depicting cultural practices used by smallholder farmers in Burkina Faso to control Striga hermonthica infestation in pearl millet fields. A = terraces to conserve soil moisture, B = ridge planting, C = use of grass strips as Striga push, and D = use of micro plots or planting holes (locally referred to as zaï) to grow healthy and vigorous pearl millet seedlings (Rouamba et al. 2021)
Chemical control
Strigolactones and related chemical compounds are methods of choice in Striga management in pearl millet and sorghum production. This method involves the use of different chemicals such as dihydrosorogoleone, sesquiterpene and kinetin (Babalola and Odhiambo 2008; Cardoso et al. 2011; Zwanenburg et al. 2016). Known as suicidal germination stimulants for parasitic plants, SLs hold promises for Striga control (Zwanenburg et al. 2016). The SL analogue MP16 reduced Striga emergence by 97% under greenhouse conditions. The Nijmegen-1 analogue rendered 40% and 60% reductions of Striga emergence in pearl millet and sorghum fields, respectively, compared to the standard chemical GR-24. Though this method has been highly successful in greenhouse trials, it is still expensive for small-scale farmers (Samejima et al. 2016; Zwanenburg et al. 2016; Kountche et al. 2019).
Biological control
Biocontrol agents are vital to controlling major crop pests and diseases. They are ecologically friendly and have added benefits to soil health compared to crop protection chemicals. (Raklami et al. 2019; Jabborova et al. 2020). Biological control method is a deliberate use of living organisms to suppress parasitic plants, plant diseases and insect pests. Herbivorous insects, microorganisms (e.g., fungi), and smothering plants are vital biocontrol agents against weeds. Fusarium oxysporum f.sp. Strigae [FOS], host-specific fungi, is highly pathogenic against S. hermonthica (Mrema et al. 2020). FOS is soil-borne and has shown immense potential to control the emergence and reproduction of Striga sp. (Zarafi et al. 2015). Plant toxic compounds such as fumonisin B1 are produced by FOS that can kill Striga plants before it penetrate the roots of their host (Elzein and Kroschel 2004; Rebeka 2007). Pathogenic fungi are host-specific, highly destructive, easy to reproduce, and genetically divergent (Ciotola et al. 2000). Rebeka (2007) and Elzein et al. (2008) reported the pathogenicity and host specificity of FOS to Striga without any adverse effects on major cereal crops (Elzein et al. 2010; Rebeka et al. 2013; Mrema et al. 2018). Planting FOS treated seeds of the host allow reproduction of the fungus in the rhizosphere of the young host plants inhibiting the growth and development of Striga plants (Rebeka 2007).
Another class of biological agents is the Arbuscular mycorrhizal (AM) fungus. The AM enhances crop performance, providing adequate protection against Striga and facilitating the assimilation of phosphorus (P), water, and micronutrients from the soil by the companion crops. The use of AM-treated maize decreased the incidence of S. hermonthica and increased plants' nitrogen (N) and P uptake (Bonfante and Genre 2010; Samejima and Sugimoto 2018). Artificial inoculation of sorghum seeds with Bacillus subtilis, B. amyloliquefaciens, and Burkholderia phytofirmans reportedly reduced Striga infestation by 47% (Mounde et al. 2015). Bacillus and Streptomyces species-derived enzymes, such as xylanases, pectinase, and amylases damaged Striga seeds.
Host plant resistance
Host resistance is the most economical approach to control Striga because resistant cultivars can be grown with limited production input (Hess and Ejeta 1992). Striga resistance is defined as the ability of the host to prevent Striga attachment and development while yielding reasonably well than the susceptible genotypes (Ramaiah 1987; Ejeta et al. 1993). Conversely, tolerance is the ability of the host to maintain high yield compared to susceptible check (Haussmann et al. 2000; Rodenburg et al. 2005; Hearne 2009). Host resistance has not been fully utilized in breeding programs due to the partial resistance conferred by major genes (Ramaiah 1987; Wilson et al. 2004; Mwangangi et al. 2021; Rouamba et al. 2022). Plants employ different mechanisms to resist and tolerate Striga infestation (Anitha et al. 2020).
Integrated Striga management
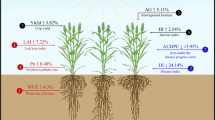
Integration of multiple control methods, also referred to as integrated Striga management (ISM) are efficient and economical to control Striga, including under smallholder farmers' conditions (Tesso et al. 2007). Figure 2 depicts tri-trophic interactions for integrated Striga control through resistant genotypes compatible with a biocontrol agent (FOS). The picture portrays the underlying mechanisms and principles of integrating the Striga-resistant genotype with FOS treatment. The system reportedly enhances the effectiveness of the biocontrol agent with ultimate yield gains in sorghum and maize. An ISM is considered the most cost-effective and environmentally friendly and can quickly be adopted by smallholder pearl millet farmers (Joel 2000; Hearne 2009).
Components of host plant resistance
Root architecture
Field resistance to Striga parasites is attributed to root architecture and physiology. Plant roots serve as mechanical barriers that may resist haustorial attachment or avoid contact with pests held in seed banks (Ejeta 2000; Gurney et al. 2003). Antibiosis and hypersensitivity to Striga infection are due to active resistance functions in the host plants barring the contact of the parasite with the host. Haustorial interference with the host's root surface causes biochemical responses that cause histological changes, such as necrosis of the host's root cells to prevent penetration of distal cells of Striga. A genotype with hypersensitive resistant mechanisms inhibits parasitic attachment and growth and deprives its access to nourishment to reach maturity (Ejeta 2000). However, the host in a given environment might succumb to one Striga population but resist another community of the same parasite (David et al. 2022). Hence, reliance on root architectural resistance alone may be insufficient and unreliable. Furthermore, host plants escape infestation by reduced root biomass production and root architecture that avoids the soil layer in which the parasite seeds are more common (Wegmann et al. 1991).
Stay-green trait
High photo-inhibition rates per unit leaf are typical on most cereals infested by Striga spp. Therefore, maintaining high photosynthetic efficiency under heavy Striga infestation is key to increasing tolerance levels to the parasite (Gurney et al. 2003). The stay-green or delayed senescence trait determines the ability of plants to keep their leaves in the active photosynthetic stage to sustain photo-assimilate production and reproductive efficiency under biotic and abiotic stress conditions. Striga damage and drought symptoms exhibit rapid leaf senescence and degeneration of leaf chlorophyll. Augmenting the stay-green trait with other Striga resistance components may increase host defence and boost yield gains. Ribaut et al. (2009) and Luche et al. (2015) reported that gains in grain yield under Striga infestation have been associated with delayed senescence. Delayed senescence is expressed in two forms, namely functional and non-functional stay green characteristics. Functional stay-green plants continue to grow under conditions that lead to senescence in the wild type (Thomas and Howarth 2000). Non-functional stay-green plants are defective in the breakdown of chlorophyll and remain green even though chloroplasts are no longer photosynthetically active (Thomas and Howarth 2000). The former is relevant to Striga tolerance as mutants with functional "stay-green" have prolonged photosynthetic activity and delayed senescence than standard genotypes. In maize, genomic regions conferring stay-green have been mapped within the genome. Three stay-green QTLs, qsg-1, qsg-4, and qsg-8, have been identified under low nitrogen conditions (Ribeiro et al. 2018), showing the importance of the stay-green trait in Striga populations. In pearl millet two stay-green QTLs (Xibmsp09/AP10.2, and Xibmcp09/AP10.1) have been identified and four markers on chromosome 6 were reportedly associated with stay-green trait (Singh and Nara 2023).
Escape
The ability of genotypes to complete the reproductive life cycle before peak pest pressure, moisture deficit or disease outbreak is defined as ‘escape’. Selection for Striga escape through early maturity can reduce yield loss due to the parasites and their derived phytotoxins (Rich and Ejeta 2008). Evaluating drought-tolerant extra early germplasm under Striga infestation is fundamental in breeding for early maturity. Ultra-early cultivars that complete their life cycle before the continuous effects of multiple Striga infestations are required where terminal drought stress jointly occurs with the parasite (Wegmann et al. 1991).
Striga resistance genes
Striga resistance is conditioned by a chain of signals elicited by the host plant. The inheritance of crop traits associated with Striga resistance are not conclusively known. Some modest success are reported in sorghum and only brief progress in pearl millet. Kountche et al. (2013) reported quantitative resistance to S. hermonthica using cultivated pearl millet gene pool under field evaluations. Pearl millet landraces such as M141, M239, M029, M197, M017, KBH, and 29Aw have been reported to possess Striga resistance genes (Kountche et al. 2013, Dayou et al. 2021). The introgression of multiple resistance genes in a single cultivar would provide more robust resistance to Striga (Kountche et al. 2016). Although conventional breeding has significantly contributed to Striga resistance, particularly in sorghum and pearl millet, this approach has not been fully deployed due to the complex quantitative resistance to Striga. Thus, the development of molecular markers offer an opportunity to identify resistant genes in wild relatives and resistant varieties of related species (Ejeta and Gressel 2007; Rispail et al. 2007). This may facilitate the pyramiding of multiple resistance genes into the agronomically superior and locally adapted Striga susceptible varieties (Kountche et al. 2016). Marker-assisted backcross has been used to introgress Striga-resistant genes from Striga-resistant lines (N13, SRN 39, Framida and Hakika) to farmers preferred lines (Gadam and Kari Mtama-1) that are susceptible to Striga in sorghum (Muchira 2022). Novel resistance genes were identified in the wild perennial maize, Zea diploperennis. The candidate genes were introgressed into early- and extra-early-maturing maize inbreds (Amegbor et al. 2017). Early‐maturing Striga-resistant and drought‐tolerant maize inbred line, TZdEI 352, derived from a cross between TZEW Pop DT STR and Z. diploperennis had increased grain yield and durable Striga resistance/tolerance (Akaogu et al. 2019).
Screening for Striga resistance in pearl millet
Several screening techniques were reported (Berner et al. 1997; Haussmann et al. 2000). These included double-pot, Pasteur pipette, root-slope, sandwich, and antihaustorial. Screening in pots requires growing the host in pots artificially inoculated with Striga seeds. Striga infestation in pots is more definite than in artificially infested fields (Rao et al. 1983). The agar-gel assay developed by Hess and Ejeta (1992) provides a relatively easy means for screening host genotypes for low Striga seed germination stimulant production. These screening techniques used in other crops can be adapted to screen pearl millet for Striga resistance breeding.
Breeding for Striga resistance
Conventional breeding
Considerable efforts have been made in breeding cereals for Striga resistance, and modest progress has been achieved in developing improved varieties (Yacoubou et al. 2021). Identifying potential sources of resistance is the first procedure of all Striga resistance breeding programmes. Crossing complementary parents with resistance genes and agronomic traits followed by recurrent selection increases the integration of Striga resistance genes. This method will build polygenic resistance, durable, and effective over time for the control of Striga (Menkir and Kling 2007). Recurrent selection has been used to develop the first experimental pearl millet Striga-resistant variety (Kounche et al. 2013). In maize breeding, Striga damage symptoms and counts were reduced by 3% and 10% per cycle of recurrent selection, and grain yield increased by 16% (Menkir et al. 2004). The half-sib as well as full-sib selection schemes are ways to develop composite populations with moderate resistance to S. hermonthica by allowing few Striga attachments compared to susceptible genotypes (Hallauer 1992; John and Sleeper 1995; Menkir et al. 2004). Conversely, the availability of donor parents with Striga resistance could facilitate the introgression of a favourable gene using backcrossing (Badu-Apraku et al. 2017).
Marker-assisted selection
Molecular marker techniques are complementary genomic resource in traditional plant breeding and genetic analysis. Marker-assisted selection (MAS) is an indirect selection procedure to identify a trait of interest (e.g., Striga resistance) based on a molecular marker linked to the phenotypic trait (Ribaut et al. 2001). MAS allow the selection of better-performing genotypes at early generations (Yacoubou et al. 2021). Using simple sequence repeats (SSRs) and single nucleotide polymorphisms (SNPs) markers, some elite genotypes for the breeding of Striga resistance were selected, and new markers have been identified, which significantly contributed to the differentiation of Striga tolerant and susceptible genotypes (Bawa et al. 2015; Shayanowako et al. 2018). Quantitative trait locus (QTL) for S. hermonthica resistance from local populations have been successfully transferred through backcross breeding into adaptable maize populations using MAS (Rich and Ejeta 2008). Striga resistance QTL were discovered in sorghum and rice (Atera et al. 2015; Yasir and Abdalla 2013; Yohannes et al. 2016; Ali et al. 2016) while SNPs markers associated with Striga emergence count were reported by Dawud et al. (2018) in pearl millet. Haussmann et al. (2004) identified and mapped QTL associated with Striga-resistance in the sorghum variety, N13, where a mechanical barrier is the suggested mechanism of Striga resistance. The identification of Striga-resistance QTL for pearl millet will ease the transfer of candidate genes into adaptable pearl millet varieties.
Developing a marker-assisted selection scheme for enhancing quantitative Striga resistance in pearl millet shortens the breeding cycle. Due to low genotyping costs (Elshire et al. 2011), more significant numbers of entries could be screened for markers linked to resistance alleles, followed by field phenotyping of a selected subset of the entries with an increased selection intensity. When the field phenotyping method successfully differentiates the tested entries, the results can be re-calibrated to have the marker-based selection index (Kountche et al. 2013).
Genetic resources of pearl millet for Striga resistance and economic traits
Landraces
Landraces are novel sources of genetic variation for breeding based on their desirable genetic compositions for agronomic and quality attributes. Many accessions of pearl millet are curated in limited gene banks and databases globally (Table 1). There is a need to screen for large numbers of memberships to identify the required and desirable germplasm and genes for breeding. The first selfed generation (S1) gene pool is more efficient for utilising landraces when limited genetic information is available. The S1 gene pools are mixtures of selfed individuals from a more significant number of accessions, allowing for a more efficient evaluation of germplasm (Burton 1978; Hanna 1990). Furthermore, the S1 gene pools allow to assess large populations and select the desired trait (s) more readily. Genetic diversity analyses in landraces offer possibilities of pearl millet breeding of open-pollinated and hybrid varieties (Langridge 2005; Varshney and Tuberosa 2007). In pearl millet (Wilson et al. 2004) and maize (Rich and Ejeta 2008), wild relative genotypes have been used as Striga resistance sources for variety development.
Mutant selections
Kiruki et al. (2006) reported the first Striga-resistant mutant maize varieties (K9908, K9910 and K9911). The varieties had stable performances in Striga-infested fields in western Kenya. A mutation at LGS1 locus causes quantitative and qualitative alterations in the SL content of root exudates, significantly reducing the germination stimulant's action without negatively impacting productivity (Gobena et al. 2017). Nikièma et al. (2020) identified seven Striga-resistant mutants (SA38M5, SA188M6, GK715M4, GK225M5, IC47M5, IC83M5 and IC17M6) among sorghum mutants generated from gamma irradiation. For the performance and estimation of the genetic variability study, M3 population of pearl millet treated with different doses of gamma rays showed high heritability for panicle diameter, number of nodes per plant and stem diameter (Maryono et al. 2020). Induced mutation is a powerful tool in pre-breeding in pearl millet to generate new breeding populations to identify Striga-resistant mutants and cultivar development.
Hybrid varieties
Hybrid varieties are man-made entities developed by crossing two genetically distant breeding lines. They represent the first generation (F1) originating from the cross. In pearl millet, hybrids are developed as follows: (i) development of inbred lines in the various original populations, (ii) test crosses between the different inbred lines to find the best hybrids, and (iii) production of hybrid seed for the market (Arncken and Dierauer 2006). Hybrid seed production requires efficient cross-pollination methods to keep production costs low (Duvick 2009). Pearl millet hybrids outperformed landraces by 10–15% (Yadav and Rai 2013). However, the new hybrids could not be adopted because of the lack of efficient seed production programs and their limited genetic superiority (Yadav et al. 2021). Hybrids are known and desirable for their high productivity and quality. However, they have shown reduced disease resistance compared to open-pollinated varieties (OPVs) with innate defence traits (Schroeder et al. 2013). It is, therefore, vital to understand the parents' genetic makeup by combining ability analyses to develop hybrids with enhanced resistance to S. hermonthica (Yacoubou et al. 2021). Pearl millet hybrid derived from crosses between S. hermonthica resistant and susceptible parents were reported to be susceptible due to the recessive genes conditioning resistance which were masked by dominant genes (Haussmann et al. 2000; Rouamba et al. 2022). Hess and Ejeta (1992) and Kling et al. (2000) reported that heterosis can offer tolerance to Striga in sorghum and maize. Maize hybrid varieties with Striga resistance have been reported by Menkir et al. (2004) and Karaya et al. (2012). This suggested that hybrid breeding can offer Striga resistance which can also be exploited in pearl millet breeding.
Synthetic varieties
A synthetic variety is derived from an open-pollinated population (Lonnquist 1949). Synthetics can be formed by inter-crossing selfed plants or lines that are subsequently maintained by mass selection. A synthetic variety designates a genetic pool derived from open-pollination or controlled crosses of all possible combinations among several genotypes subjected to a combining ability test. The progenitors of a synthetic variety could be inbred or mass-selected populations (Mandal 2014). The merit of synthetics has been observed in sorghum cultivars and demonstrated an average superiority of 18% for grain yield under Striga infestation (Haussmann et al. 2000). Host plant damage was significantly reduced in synthetic maize populations resistant to Striga (Kim et al. 1998). Synthetic varieties partially utilize heterosis because some inbreeding occurs to open pollination in later generations (Mohammed 2013). Being a cross-pollinated crop, developing pearl millet synthetic variety with Striga resistance may contribute to Striga-resistance stability over time.
Genomic-assisted breeding
Quantitative trait loci (QTL) analysis
Quantitative traits are useful to plant breeders. Most of the economic traits have quantitative inheritance. A QTL is a region on the genome that may comprise one or more functional genes. In maize, the resistance to S. hermonthica is regulated by polygenes or QTL with small additive genetic contributions (Rodenburg et al. 2006; Shayanowako et al. 2020). QTL related to Striga damage rating and Striga emergence count have been identified in maize by Badu-Apraku et al. (2020), including qepp-3, qepp-8.1, qsd-5.1, and qsc-3.1. Identifying QTL associated with Striga-resistance facilitate the rapid development of Striga-resistant pearl millet genotypes using MAS. The polygenic nature of host–parasite relationship and its interaction with environmental factors after validation necessitate the use of MAS (Gedil and Menkir 2019). No significant research and development has been done on the detection of QTL or minor genes for Striga resistance in SSA (Yacoubou et al. 2021). MAS used in maize may serve as a model tool in pearl millet Striga resistance breeding programs.
Next-generation sequencing (NGS)
Next-generation and conventional sequencing technology have been used to elucidate the molecular events underlying Striga resistance (Yoshida et al. 2010). Striga genomes have a typical complex angiosperm genome with a size of 615 Mb for S. asiatica,1425 Mb for S. hermonthica and 2460 Mb for S. forbesii, suggesting several polyploidization events (Schneeweiss et al. 2004). Next-generation sequencing technology has increased available transcriptional data for S. hermonthica and related species (Spallek et al. 2013).
Genomics-assisted breeding is one of the most promising developments that have implications for imparting genetic gains in pearl millet breeding. Genomic selection improves the breeding program's precision and efficiency (Yadav et al. 2021). Through whole-genome resequencing of Pearl Millet Inbred Germplasm Association Panel, mapping population parents, and elite hybrid parental lines more than > 32 million repositories of genome-wide SNPs were developed (Varshney et al. 2017). The genomic and genetic resources enable the development of genetic maps and rapidly deploying genes of agronomic importance. Also, it allows resequencing lines to mine and map genes of interest in pearl millet (Yadav et al. 2021). NGS based on the repository of genome-wide SNPs could substantially accelerate knowledge in Striga-resistance breeding to deliver pearl millet varieties with Striga resistance and farmers’ preferred traits. The inherent biases and ambiguous alignment of repetitive genetic and nongenetic elements lead to highly fragmented draft genome assemblies that may hinder the use of NGS and complicate studies of hidden indels and structural variants (Sedlazeck et al. 2018). Gobena et al. (2017) reported transcriptome data on S. hermonthica plants of different development stages through NGS analysis. A new gene (WKRY45) in rice and an RNA-seq in finger millet were reported to be associated with Striga resistance (Yoshida and Shirasu 2012; Mutuku et al. 2015).
Genetic engineering and genome editing
Genetic engineering
Genetic engineering involves integrating genetic material through transformation followed by selection. Genetic engineering permits the transfer of resistance genes from any organism into a reference crop. Genetic engineering can be deployed to integrate resistance genes against Striga, including the strigolactone content of the host plant. Genetic resistance can either be adopted solo or as part of an integrated management system (Jamil et al. 2021; Kavuluko et al. 2021; Muchira et al. 2021; Mallu et al. 2022). In Striga resistance breeding, the main limitation to employing genetic engineering is lack of well-defined resistance genes (Haussmann et al. 2000). The RNA interference (RNAi) technology has been explored as a genetic tool for engineering host plants with resistance against parasitic weeds. The RNAi technology can transform host plants with a plasmid encoding a double-stranded hairpin RNA (hpRNA) targeted against one or more Striga resistance genes (Runo et al. 2011; Yoder et al. 2009).
Genome editing
Genome editing (also referred to as gene editing) is a set of tools enabling editing genes to enhance the genetic expression of an organism. It manipulates the specific gene loci to gain genome modifications, such as insertions, deletions or point mutations. Genome editing techniques were developed in the late 1990s with the discovery of homing and zinc-finger endonucleases, which direct DNA cleavage to particular sites within a genome. The three main genome editing tools currently used are ZFNs, TALENs, and CRISPR/Cas9 (ASSAF 2016). CRISPR-Cas9 is the most accurate and efficient genome editing technique (Barrangou 2015). Butt et al. (2018) reported that CRISPR/Cas9 system in translational research can be used for target improvement of plant architectural trait. The study showed that targeted engineering of CCD7 could improve crop yield and lower the risk of Striga infestation by increasing the number of tillers while significantly reducing Striga germination in rice.
Limitations of the reviewed studies and potential sources of bias
Research efforts in S. hermonthica resistance breeding in pearl millet are limited compared to other major cereal crops (Mudereri et al. 2020; Stanley et al. 2021). Dossa et al. (2023) conducted a meta-analysis and summarised the most effective methods for Striga control. However, the authors only found one report for pearl millet and finger millet compared to 46 and 18 for maize and sorghum, respectively. The sparse studies available on pearl millet and finger millet could hinder drawing plausible conclusions of Striga resistance breeding efforts of the two important crops. Therefore, data presented based on the findings of maize and sorghum might be a source of bias until more research is conducted and robust data presented revealing the interaction of pearl millet and Striga to guide breeding and genetic analysis. The wide range of hosts of S. hermonthica, including primary hosts (e.g., cereal crops) and alternative hosts (e.g., grasses) may complicate its management and control methods. Genomic resources and innovations can reveal the molecular and genetic bases of host resistance and host–parasite interaction for the precision of pearl millet with durable Striga resistance (Jamil et al. 2021). Further, new genetic and genomic resources and safe and sustainable control strategies, including beneficial microorganisms, should be explored to control the scourge of Striga infestation (Olowe et al. 2023).
Conclusion and outlook
Pearl millet yield in SSA is low due to various biotic and abiotic factors. Striga causes yield loss of up to 100% in heavily infested fields. Cultural practices, chemical and biocontrol agent control measures are recommended for Striga management. However, the methods were not widely adopted by smallholder farmers because of their unavailability or high cost and Striga’s complex life cycle and prolonged seed dormancy in farmlands. Striga resistance varieties are cost-effective, environmentally friendly and affordable for smallholder farmers to control and reduce Striga infestations and improve pearl millet yields. Furthermore, integrated Striga management involving pearl millet genotypes with Striga-resistance and FOS compatibility is the most cost-effective, and environmentally friendly and can quickly be adopted by smallholder pearl millet farmers. Information presented in this review, including genetic resources, new breeding technologies, and innovations, assists in the precision and speed breeding of Striga-resistant cultivars. Overall, the review will guide the use of the best breeding strategies and accelerate the development of new pearl millet cultivars that are high yielding and resistant to S. hermonthica to reduce damage incurred by Striga infestations on farmers’ fields in Burkina Faso and similar agro-ecologies. There is a need for training pearl millet farmers about the occurrence, distribution, and management of S, hermonthica. Furthermore, pearl millet is an under-researched crop needing research and development priority and policymakers' support to enhance the crop's production and productivity through breeding Striga-resistant varieties using new genetic and genomic resources.
Availability of data and materials
Not applicable.
Abbreviations
- AM:
-
Arbuscular mycorrhizal
- CGR:
-
Canadian genetic resources
- FOS :
-
Fusarium oxysporum F.sp. Strigae
- GRIN:
-
Germplasm Resource Information Network
- IRD:
-
Institute of Research for Development
- ISM:
-
Integrated Striga management
- ICRISAT:
-
International Crops Research Institute for the Semi-Arid Tropics
- MAS:
-
Marker-assisted selection
- NGS:
-
Next-generation sequencing
- OPVs:
-
Open-pollinated varieties
- QTL:
-
Quantitative trait loci
- SSRs:
-
Simple sequence repeats
- SNPs:
-
Single nucleotide polymorphisms
- SADC:
-
Southern African Development Community
- SLs:
-
Strigolactones
- SSA:
-
Sub-Saharan Africa
References
Akaogu IC, Badu-Apraku B, Tongoona P, Ceballos H, Gracen V, Offei SK, Dzidzienyo D. Inheritance of Striga hermonthica adaptive traits in an early-maturing white maize inbred line containing resistance genes from Zea diploperennis. Plant Breeding. 2019;138(5):546–52.
Al-Babili S, Bouwmeester HJ. Strigolactones, a novel carotenoid-derived plant hormone. Annu Rev Plant Biol. 2015;66:161–86.
Ali R, Hash CT, Damaris O, Elhussein A, Mohamed AH. Introgression of Striga resistance into popular Sudanese sorghum varieties using marker assisted selection. World J Biotechnol. 2016;1:48–55.
Ali RA, El-Hussein AA, Mohamed KI, Babiker AGT. Specificity and genetic relatedness among Striga hermonthica strains in Sudan. Int J Life Sci. 2009;3:1159–66.
Amegbor IK, Badu-Apraku B, Annor B. Combining ability and heterotic patterns of extra-early maturing white maize inbreds with genes from Zea diploperrennis under multiple environments. Euphytica. 2017;213:1–16.
Anitha K, Das I, Holajjer P, Sivaraj N, Reddy CR, Balijepalli SB. Sorghum diseases: diagnosis and management. In: Tonapi VA, Talwar HS, Are AK, Bhat BV, Reddy CR, Dalton TJ, editors. Sorghum in the 21st century food fodder feed fuel for a rapidly changing world. Singapore: Springer; 2020. p. 565–619.
Arncken C, Dierauer H. Hybrid varieties for organic cereals? Prospects and acceptance of hybrid breeding for organic production 2006.
ASSAF. The regulatory implications of new breeding techniques 2016.
Atera EA, Onyango JC, Thanh PT, Ishii T, Itoh K. Identification of QTL for Striga hermonthica resistance using backcross population derived from a cross between Oryza sativa (cv. Nipponbare) and O. rufipogon. J Agric Sci. 2015;7(2):99–105.
Babalola OO, Odhiambo GD. Effect of inoculation with Klebsiella oxytoca ’10 mkr 7’on Striga suicidal germination in Zea mays. World Appl Sci J. 2008;3(1):57–62.
Badu-Apraku B, Fakorede MAB, Akinwale RO. Key challenges in maize breeding in sub-Saharan Africa. Cambridge: Burleigh Dodds Science Publishing; 2017. p. 51–86.
Badu-Apraku B, Adewale S, Paterne A, Gedil M, Asiedu R. Identification of QTLs controlling resistance/tolerance to Striga hermonthica in an extra-early maturing yellow maize population. Agronomy. 2020;10:1168.
Baiyegunhi LJS, Hassan MB, Danso-Abbeam G, Ortmann GF. Diffusion and adoption of Integrated Striga Management (ISM) technologies among smallholder maize farmers in rural northern Nigeria. Technol Soc. 2019;56:109–15.
Barrangou R. The roles of CRISPR–Cas systems in adaptive immunity and beyond. Curr Opin Immunol. 2015;32:36–41.
Bawa A, Abdulai MS, Addai IK. Evaluation of inbred lines and hybrid maize (Zea mays L.) for tolerance to Striga hermonthica (Del.) Benth in the guinea savanna agro-ecological zone of Ghana. Am J Agric Biol Sci. 2015;10(3):128–36.
Begna T. Effect of Striga species on sorghum (Sorghum bicolor L. Moench) production and its integrated management approaches. Int J Res Stud Agric Sci. 2021;7(7):10–22.
Belay F. Breeding sorghum for Striga resistance: a review. J Nat Sci Res. 2018;8(5):1–8.
Berner D, Winslow M, Awad A, Cardwell K, Raj D, Kim S. Striga research methods: a manual. Ibadan: The Pan-African Striga Control Network; 1997.
Bonfante P, Genre A. Mechanisms underlying beneficial plant–fungus interactions in mycorrhizal symbiosis. Nat Commun. 2010;1:1–11.
Burton GW. Registration of Tift# 1 S-1 pearl millet germplasm 1 (Reg. No. GP 9). Crop Sci. 1978;18:697–697.
Butt H, Jamil M, Wang JY, Al-Babili S, Mahfouz M. Engineering plant architecture via CRISPR/Cas9-mediated alteration of strigolactone biosynthesis. BMC Plant Biol. 2018. https://doi.org/10.1186/s12870-018-1387-1.
Cardoso C, Ruyter-Spira C, Bouwmeester HJ. Strigolactones and root infestation by plant-parasitic Striga, Orobanche and Phelipanche Spp. Plant Sci. 2011;180(3):414–20.
Cimmino A, Masi M, Rubiales D, Evidente A, Fernández-Aparicio M. Allelopathy for parasitic plant management. Nat Prod Commun. 2018;13:289–94.
Ciotola M, DiTommaso A, Watson AK. Chlamydospore production, inoculation methods and pathogenicity of Fusarium oxysporum M12–4A, a biocontrol for Striga hermonthica. Biocontrol Sci Tech. 2000;10(2):129–45.
Dafaallah AB. Biology and physiology of witchweed (Striga spp.): a review. Int J Acad Multidiscip. 2019;3:42–51.
David OG, Ayangbenro AS, Odhiambo JJ, Babalola OO. Striga hermonthica: a highly destructive pathogen in maize production. Environ Chall. 2022;8:100590.
Dawud MA. Striga resistance in cereal crops: recent progress and future prospects. A review. Glob J Sci Front Res. 2017;17:1.
Dawud MA. Genetic Studies of Pearl Millet (Pennisetum Glaucum [L.] R. Br. ) for resistance to Striga Hermonthica. PhD Thesis, University of Ghana, 2018; p. 168. http://ugspace.ug.edu.gh.
De Groote H, Wangare L, Kanampiu F, Odendo M, Friesen D. Potential markets for herbicide resistant maize seed for Striga control in Africa 2005;(No. 724-2016-49107).
Dayou O, Kibet W, Ojola P, Gangashetty PI, Wicke S, Runo S. Two-tier witchweed (Striga hermonthica) resistance in wild pearl millet (Pennisetum glaucum) 29Aw. Weed Sci. 2021;69(3):300–6.
Dossa EN, Shimelis H, Shayanowako AI, Laing MD. A meta-analysis of the effects of Striga control methods on maize, sorghum, and major millets production in sub-Saharan Africa. Crop Sci. 2023;63(2):460–79.
Drabo I, Zangre RG, Danquah EY, Ofori K, Witcombe JR, Hash CT. Identifying famers’ preferences and constraints to Pearl Millet production in the Sahel and North-Sudan zones of Burkina Faso. Exp Agric. 2018;55(5):765–75.
Duvick DN. Selection methods part 3: hybrid breeding. In: Ceccarelli S, Guimarães EP, Weltzien E, editors. Plant breeding and farmer participation. Rome: Food and Agriculture Organization of the United Nations; 2009. p. 229–53.
Ejeta G, Butler L, Babiker AGT. New approaches to the control of Striga: Striga Research at Purdue University Research Bulletin No. 991. Agricultural Experimental Station, 1993;Purdue University, West Lafayette.
Ejeta G, Gressel J. Integrating new technologies for Striga control: towards ending the witch-hunt. Singapore: World Scientific; 2007.
Ejeta G. Molecular mapping of Striga resistance genes in sorghum. In: Haussmann BIG, Hess DE, Koyama ML, Grivet L, Rattunde HFW, Geiger HH (Eds.), Breeding for Striga, 2000.
Elshire RJ, Glaubitz JC, Sun Q, Poland JA, Kawamoto K, Buckler ES, Mitchell SE. A robust, simple genotyping-by-sequencing (GBS) approach for high diversity species. PLoS ONE. 2011;6(5):e19379.
Elzein A, Kroschel J. Fusarium Oxysporum foxy 2 shows potential to control both Striga Hermonthica and S. Asiatica. Weed Res. 2004;44:433–8.
Elzein A, Brändle F, Cadisch G, Kroschel J, Marley P, Thines M. Fusarium oxysporum strains as potential Striga mycoherbicides: molecular characterization and evidence for a new forma specialis. Open Mycol J. 2008;2(1):89.
Elzein A, Heller A, Ndambi B, De Mol M, Kroschel J, Cadisch G. Cytological investigations on colonization of sorghum roots by the mycoherbicide Fusarium oxysporum f. sp. Strigae and its implications for Striga control using a seed treatment delivery system. Biol Control. 2010;53(3):249–57.
FAOSTAT 2018. Food and Agriculture Organization of the United Nations. Rome Italy. 2018. http://www.fao.org/ Accessed 20 Jun 2020.
FAOSTAT 2022. FAOSTAT online database 2022: Rome Italy, 2022.
Franke A, Van den Brand G, Vanlauwe B, Giller K. Sustainable intensification through rotations with grain legumes in sub-Saharan Africa: a review. Agr Ecosyst Environ. 2018;261:172–85.
Gedil M, Menkir A. An integrated molecular and conventional breeding scheme for enhancing genetic gain in maize in Africa. Front Plant Sci. 2019;10:1430.
Ghatak A, Chaturvedi P, Nagler M, Roustan V, Lyon D, Bachmann G, Weckwerth W. Comprehensive tissue-specific proteome analysis of drought stress responses in Pennisetum glaucum (L.) R. Br.(Pearl millet). J Proteomics. 2016;143:122–35.
Gobena D, Shimels M, Rich PJ, Ruyter-Spira C, Bouwmeester H, Kanuganti S, Ejeta G. Mutation in sorghum low germination stimulant 1 alters strigolactones and causes Striga resistance. Proc Natl Acad Sci. 2017;114(17):4471–6.
Goldwasser Y, Rodenburg J. Integrated agronomic management of parasitic weed seed banks. In: Joel DM, Gressel J, Musselman LJ, editors. Parasitic orobanchaceae: parasitic mechanisms and control strategies. New York: Springer; 2013. p. 393–413.
Goyet V, Wada S, Cui S, Wakatake T, Shirasu K, Montiel G, Yoshida S. Haustorium inducing factors for parasitic Orobanchaceae. Front Plant Sci. 2019;10:1056.
Gurney AL, Grimanelli D, Kanampiu F, Hoisington D, Scholes JD, Press MC. Novel sources of resistance to Striga hermonthica in Tripsacum dactyloides, a wild relative of maize. New Phytol. 2003;160(3):557–68.
Hallauer AR. Recurrent selection in maize. Plant Breeding Rev. 1992;9:115–79.
Hanna WW. Registration of Tift 2 S-1 pearl millet germplasm. Crop Sci. 1990;30(6):1376.
Hassan MB, Ortmann GF, Baiyegunhi LJS. Impact of integrated Striga management (ISM) technology on maize productivity in Northern Nigeria: a treatment effect approach. Afr J Sci Technol Innov Dev. 2018;10(3):335–44.
Haussmann BI, Hess DE, Welz HG, Geiger HH. Improved methodologies for breeding Striga-resistant sorghums. Field Crop Res. 2000;66(3):195–211.
Haussmann BI, Hess DE, Omanya G, Folkertsma R, Reddy B, Kayentao M, Welz HG, Geiger HH. Genomic regions influencing resistance to the parasitic weed Striga hermonthica in two recombinant inbred populations of sorghum. Theor Appl Genet. 2004;109(5):1005–16.
Hearne SJ. Control—the Striga conundrum. Pest Manag Sci. 2009;65(5):603–14.
Hess DE, Ejeta G. Inheritance of resistance to Striga in sorghum genotype SRN39. Plant Breeding. 1992;109:233–41.
Hood ME, Condon JM, Timko MP, Riopel JL. Primary haustorial development of Striga asiatica on host and nonhost species. Phytopathology. 1998;88(1):70–5.
INSD. Annuaire statistique 2020, 2021.
Jabborova D, Wirth S, Kannepalli A, Narimanov A, Desouky S, Davranov K, Sayyed RZ, El Enshasy H, Malek RA, Syed A. Co-inoculation of rhizobacteria and biochar application improves growth and nutrientsin soybean and enriches soil nutrients and enzymes. Agronomy. 2020;10:1142.
Jamil M, Kountche BA, Al-Babili S. Current progress in Striga management. Plant Physiol. 2021;185:1339–52.
Jamil M, Wang JY, Yonli D, Ota T, Berqdar L, Traore H, Margueritte O, Zwanenburg B, Asami T, Al-Babili S. Striga hermonthica suicidal germination activity of potent strigolactone analogs: evaluation from laboratory bioassays to field trials. Plants. 2022;11:1045.
Joel DM. The long-term approach to parasitic weeds control: manipulation of specific developmental mechanisms of the parasite. Crop Prot. 2000;19(8–10):753–8.
Joel DM, Bar H. The seed and the seedling. In: Joel DM, Gressel J, Musselman LJ, editors. Parasitic orobanchaceae: parasitic mechanisms and control strategies. New York: Springer; 2013. p. 147–65.
John M, Sleeper D. Breeding field crops. Ames: Iowa State University Press; 1995.
Kamara AY, Menkir A, Chikoye D, Tofa AI, Fagge AA, Dahiru R, Solomon R, Ademulegun T, Omoigui L, Aliyu KT, Kamai N. Mitigating Striga hermonthica parasitism and damage in maize using soybean rotation, nitrogen application, and Striga-resistant varieties in the Nigerian savannas. Exp Agric. 2020;56:620–32.
Kanampiu F, Makumbi D, Mageto E, Omanya G, Waruingi S, Musyoka P, Ransom J. Assessment of management options on Striga infestation and maize grain yield in Kenya. Weed Sci. 2018;66(4):516–24.
Karaya H, Kiarie N, Mugo S, Kanampiu F, Ariga E, Nderitu J. Identification of new maize inbred lines with resistance to Striga hermonthica (Del.) Benth. J Crop Prot. 2012;2:131–42.
Kavuluko J, Kibe M, Sugut I, Kibet W, Masanga J, Mutinda S, Wamalwa M, Magomere T, Odeny D, Runo S. GWAS provides biological insights into mechanisms of the parasitic plant (Striga) resistance in sorghum. BMC Plant Biol. 2021. https://doi.org/10.1186/s12870-021-03155-7.
Khan ZR, Midega CA, Bruce TJ, Hooper AM, Pickett JA. Exploiting phytochemicals for developing a ‘push–pull’crop protection strategy for cereal farmers in Africa. J Exp Bot. 2010;61(15):4185–96.
Kim SK, Akintunde AY, Walker P. Responses of maize, sorghum and millet host plants to infestation by Striga hermonthica. Crop Prot. 1994;13(8):582–90.
Kim SK, Fajemisin JM, Thé C, Adepoju A, Kling J, Badu-Apraku B, Lagoke STO. Development of synthetic maize populations for resistance to Striga hermonthica. Plant Breeding. 1998;117(3):203–9.
Kiruki S, Onek LA, Limo M. Azide-based mutagenesis suppresses Striga hermonthica seed germination and parasitism on maize varieties. Afr J Biotechnol. 2006;5(10):866.
Kling J, Fajemisin JM, Badu-Apraku B, Diallo A, Menkir A, Melake-Berhan A. Striga resistance breeding in maize. In: Haussmann, BIG, Hess DE, Koyama ML, Grivet L, Rattunde HFW, Geiger HH (Eds.), Breeding for Striga resistance in cereals. Proceedings of a Workshop, IITA, Ibadan, Nigeria, 18±20 August 1999. Margraf, Weikersheim, Germany, 2000. p. 103–118.
Kountche BA, Hash CT, Dodo H, Laoualy O, Sanogo MD, Timbeli A, Haussmann BI. Development of a pearl millet Striga-resistant genepool: response to five cycles of recurrent selection under Striga-infested field conditions in West Africa. Field Crops Res. 2013;154:82–90.
Kountche BA, Al-Babili S, Haussmann BI. Striga: a persistent problem on millets. In: Das IK, Padmaja PG, editors. Biotic stress resistance in millets. Cambridge: Academic Press; 2016. p. 173–203.
Kountche BA, Jamil M, Yonli D, Nikiema MP, Blanco-Ania D, Asami T, Zwanenburg B, Al-Babili S. Suicidal germination as a control strategy for Striga hermonthica (Benth.) in smallholder farms of sub-Saharan Africa. Plants People Planet. 2019;1(2):107–18.
Kuyah S, Sileshi GW, Nkurunziza L, Chirinda N, Ndayisaba PC, Dimobe K, Öborn I. Innovative agronomic practices for sustainable intensification in sub-Saharan Africa. A review. Agron Sustain Dev. 2021;41:1–21.
Langridge P. Molecular breeding of wheat and barley. In Proceedings of the International Congress: In the Wake of the Double Helix: From the Green Revolution to the Gene Revolution 2005; (pp. 279–286). Bologna, Italy: Avenue media.
Lee N, Thierfelder C. Weed control under conservation agriculture in dryland smallholder farming systems of southern Africa. Agron Sustain Dev. 2017;37:1–25.
Lobulu J, Shimelis H, Laing M, Mushongi AA. Maize production constraints, traits preference and current Striga control options in western Tanzania: farmers’ consultation and implications for breeding. Acta Agric Scandinavica Sect B Soil Plant Sci. 2019;69(8):734–46.
Lonnquist JH. The development and performance of synthetic varieties of corn. Agron J. 1949;41:153–6. https://doi.org/10.2134/agronj1949.00021962004100040005x.
Luche HDS, Silva JAGD, Maia LCD, Oliveira ACD. Stay-green: a potentiality in plant breeding. Ciência Rural. 2015;45:1755–60.
Magallon-Servín P, Antoun H, Taktek S, Bashan Y, de Bashan L. The maize mycorrhizosphere as a source for isolation of arbuscular mycorrhizae-compatible phosphate rock-solubilizing bacteria. Plant Soil. 2020;451:169–86.
Mahuku G, Wosula E, Kanampiu F. Integrated Pest Management in tropical cereal crops. In: Rapisarda C, Cocuzza GEM, editors. Integrated pest management in tropical regions. Wallingford: CAB International; 2017. p. 47–74.
Mallu TS, Mutinda S, Githiri SM, Achieng Odeny D, Runo S. New pre-attachment Striga resistant sorghum adapted to African agro-ecologies. Pest Manag Sci. 2021;77(6):2894–902.
Mallu TS, Irafasha G, Mutinda S, Owuor E, Githiri SM, Odeny DA, Runo S. Mechanisms of pre-attachment Striga resistance in sorghum through genome-wide association studies. Mol Genet Genomics. 2022;297(3):751–62.
Mandal B. Maize breeding and seed production manual. DRP Korea: Food and Agriculture Organization of the United Nations, Office of the Food and Agriculture Organization in DPR Korea. 2014.
Mandumbu R, Mutengwa C, Mabasa S, Mwenje E. Challenges to the exploitation of host plant resistance for Striga management in cereals and legumes by farmers in sub-Saharan Africa: a review. Acta Agric Scandinavica Sect B Soil Plant Sci. 2019;69(1):82–8.
Maryono MY, Indriatama WM, Human S. Performance and estimation genetic variability of M3 pearl millet (Pennisetum glaucum) populations. IOP Conf Ser Earth Environ Sci. 2020;484(1):012021.
Mathur PN. Global strategy for the ex situ conservation of pearl millet and its wild relatives. Global Crop Diversity Trust, 2012; Rome, Italy.
Mbuvi D, Masiga C, Kuria E, Masanga J, Wamalwa M, Mohamed A, Odeny D, Hamza N, Timko M, Runo S. Novel sources of witchweed (Striga) resistance from wild sorghum accessions. Front Plant Sci. 2017;8:116.
Mbwika JM, Odame H, Ngungi EK. Feasibility study on Striga control in sorghum. Nairobi, African Agricultural Technology Foundation. Majestic printing works, Nairobi, Kenya. 2011. p. 78.
Menkir A, King JG. Response to recurrent selection for resistance to Striga hermonthica (Del.) Benth in a tropical maize population. Crop Sci. 2007;47:674–84.
Menkir A, Kling JG, Badu-Apraku B, Thé C, Ibikunle O. Recent advances in breeding maize for resistance to Striga hermonthica (del.) Benth. In Integrated Approaches to Higher Maize Productivity in the New Millennium: Proceedings of the Seventh Eastern and Southern Africa Regional Maize Conference. CIMMYT, 2004.
Menkir A, Crossa J, Meseka S, Bossey B, Muhyideen O, Riberio PF, Coulibaly M, Yacoubou AM, Olaoye G, Haruna A. Stacking tolerance to drought and resistance to a parasitic weed in tropical hybrid maize for enhancing resilience to stress combinations. Front Plant Sci. 2020;11:166.
Mohammed AH. Breeding to derive new suitable maize (Zea mays L). Variety for spring season. J Tikrit Univ Agric Sci. 2013;13:107.
Monyo ES. 15 years of pearl millet improvement in the SADC region. Int. Sorghum Millets Newsl. 1998;39:17–33.
Mounde LG, Boh MY, Cotter M, Rasche F. Potential of rhizobacteria for promoting sorghum growth and suppressing Striga hermonthica development. J Plant Dis Prot. 2015;122:100–6.
Mrema E, Shimelis H, Laing M, Bucheyeki T. Farmers’ perceptions of sorghum production constraints and Striga control practices in semi-arid areas of Tanzania. Int J Pest Manag. 2017;63(2):146–56.
Mrema E, Shimelis H, Laing M, Mwadzingeni L. Genetic analysis of the maximum germination distance of Striga under Fusarium oxysporum f. sp. Strigae biocontrol in sorghum. J Integr Agric. 2018;17(7):1585–93.
Mrema E, Shimelis H, Laing M. Combining ability of yield and yield components among Fusarium oxysporum f. sp. Strigae-compatible and Striga-resistant sorghum genotypes. Acta Agric Scandinavica Sect B Soil Plant Sci. 2020;70(2):95–108.
Muchira N, Ngugi K, Wamalwa LN, Avosa M, Chepkorir W, Manyasa E, Nyamongo D, Odeny DA. Genotypic variation in cultivated and wild sorghum genotypes in response to Striga hermonthica infestation. Front Plant Sci. 2021;12:671984.
Muchira NW. Genotypic Response to Striga (Striga Hermonthica) Infestation in Wild Relatives and Landraces of Sorghum (Sorghum Bicolor) and the Introgression of the Resistance Into Cultivated Varieties (Doctoral dissertation, University of Nairobi), 2022; p 126.
Mudereri BT, Dube T, Niassy S, Kimathi E, Landmann T, Khan Z, Abdel-Rahman EM. Is it possible to discern Striga weed (Striga hermonthica) infestation levels in maize agro-ecological systems using in-situ spectroscopy? Int J Appl Earth Obs Geoinf. 2020;85:102008.
Murage AW, Obare G, Chianu J, Amudavi DM, Pickett J, Khan ZR. Duration analysis of technology adoption effects of dissemination pathways: a case of ‘push-pull’ technology for control of Striga weeds and stemborers in Western Kenya. Crop Prot. 2011;30:531–8.
Mutuku JM, Yoshida S, Shimizu T, Ichihashi Y, Wakatake T, Takahashi A, Seo M, Shirasu K. The WRKY45- dependent signaling pathway is required for resistance against Striga hermonthica parasitism. Plant Physiol. 2015;168(3):1152–63.
Mutyambai DM, Bass E, Luttermoser T, Poveda K, Midega CAO, Khan ZR, Kessler A. More than “push ” and “pull ”? Plant-soil feedbacks of maize companion cropping increase chemical plant defenses against herbivores. Front Ecol Evol. 2019;7:217.
Mwangangi IM, Büchi L, Haefele SM, Bastiaans L, Runo S, Rodenburg J. Combining host plant defence with targeted nutrition: key to durable control of hemiparasitic Striga in cereals in sub-Saharan Africa? New Phytol. 2021;230:2164–78.
Ndayisaba PC, Kuyah S, Midega CAO, Mwangi PN, Khan ZR. Push-pull technology improves maize grain yield and total aboveground biomass in maize-based systems in Western Kenya. Field Crop Res. 2020;256:107911.
Niassy S, Agbodzavu MK, Mudereri BT, Kamalongo D, Ligowe I, Hailu G, Kimathi E, Jere Z, Ochatum N, Pittchar J, Kassie M, Khan Z. Performance of push–pull technology in low-fertility soils under conventional and conservation agriculture farming systems in Malawi. Sustainability. 2022;14:2162.
Nikièma MP, Yonli D, Rabefiraisana HJ, Ali A, Ouédraogo N, Traoré H, Abdelbagi MAG. Induced resistance to Striga hermonthica in sorghum by gamma irradiation. Am J Plant Sci. 2020;11(10):1545–61.
Olowe OM, Akanmu AO, Ayangbenro AS, Fadiji AE, Bitire TD, Odhiambo JJ, Babalola OO. Trenchant microbiological-based approach for the control of Striga: current practices and future prospects. Front Sustain Food Syst. 2023;7:1073339.
Owheruo JO, Ifesan BO, Kolawole AO. Physicochemical properties of malted finger millet (Eleusine coracana) and pearl millet (Pennisetum glaucum). Food Sci Nutr. 2019;7(2):476–82.
Parker C. parasiticweeds: a world challenge. Weed Sci. 2012;60:269–76.
Raklami A, Bechtaoui N, Tahiri AI, Anli M, Meddich A, Oufdou K. Use of rhizobacteria and Mycorrhizae consortium in the open field as a strategy for improving crop nutrition, productivity and soil fertility. Front Microbiol. 2019;10:1106.
Ramaiah K. Breeding cereal grains for resistance to witchweed. In: Musselman LJ, editor. Parasitic weeds in agriculture. Boca Raton: CRC Press; 1987. p. 227–42.
Randrianjafizanaka MT, Autfray P, Andrianaivo AP, Ramonta IR, Rodenburg J. Combined effects of cover crops, mulch, zero-tillage and resistant varieties on Striga asiatica (L.) Kuntze in rice-maize rotation systems. Agric Ecosyst Environ. 2018;256:23–33.
Rao MV, Chidley VL, Ramaiah KV, House LR. Breeding sorghum with resistance to Striga asiatica (L) Kuntze at ICRISAT Center 1983.
Rebeka G, Shimelis H, Laing MD, Tongoona P, Mandefro N. Evaluation of sorghum genotypes compatibility with Fusarium oxysporum under Striga infestation. Crop Sci. 2013;53(2):385–93.
Rebeka GT. Survey of pathogenic fungi on Striga in north Shewa, Ethiopia and assessment for their bio-control potential. MS diss., Haramaya University, Haramaya, Ethiopia 2007.
Ribaut JM, William HM, Khairallah M, Worland AJ, Hoisington D. Genetic basis of physiological traits. In: Reynolds MP, Ortiz-Monasterio JI, McNab A, editors. Application of physiology in wheat breeding. El Batán: CIMMYT; 2001.
Ribaut JM, Betran J, Monneveux P, Setter T. Drought tolerance in maize. In: Bennetzen JL, Hake SC, editors. Handbook of maize: its biology. New York: Springer; 2009. p. 311–44.
Ribeiro PF, Badu-Apraku B, Gracen VE, Danquah EY, Garcia-Oliveira AL, Asante MD. … Gedil M Identification of quantitative trait loci for grain yield and other traits in tropical maize under high and low soil-nitrogen environments. Crop Sci. 2018;58(1):321–31.
Rich PJ. Genetic and management options for controlling Striga. In: Tonapi VA, Talwar HS, Are AK, Bhat BV, Reddy CR, Dalton TJ, editors. Sorghum in the 21st Century: food–fodder–feed–fuel for a rapidly changing world. Singapore: Springer Singapore; 2020. p. 421–51.
Rich PJ, Ejeta G. Towards effective resistance to Striga in African maize. Plant Signal Behav Behav. 2008;3:618–21.
Rispail N, Dita MA, González-Verdejo C, Pérez-de-Luque A, Castillejo MA, Prats E, Rubiales D. Plant resistance to parasitic plants: molecular approaches to an old foe. New Phytol. 2007;173(4):703–12.
Rodenburg J, Bastiaans L, Weltzien E, Hess DE. How can field selection for Striga resistance and tolerance in sorghum be improved? Field Crop Res. 2005;93(1):34–50.
Rodenburg J, Bastiaans L, Krop MJ, van Ast A. Effects of host plant genotype and seed bank density on Striga reproduction. Weed Res. 2006;46:251–63.
Rodenburg J, Riches CR, Kayeke JM. Addressing current and future problems of parasitic weeds in rice. Crop Prot. 2010;29:210–21.
Rodenburg J, Demont M, Zwart SJ, Bastiaans L. Parasitic weed incidence and related economic losses in rice in Africa. Agr Ecosyst Environ. 2016;235:306–17.
Roger ZG, Ramaiah KV. Screening of pearl millet cultivars for resistance to Striga hermonthica. In: Proceedings of the Second International Workshop on Striga, Ouagadougou, Upper Volta; 1981. p. 77–81.
Rouamba A, Shimelis H, Drabo I, Laing M, Gangashetty P, Mathew I, Mrema E, Shayanowako AIT. Constraints to pearl millet (Pennisetum glaucum) production and farmers’ approaches to Striga hermonthica management in Burkina Faso. Sustainability. 2021;13:8460.
Rouamba A, Shimelis H, Drabo I, Shayanowako AIT, Mrema E, Gangashetty PI. Generation mean analysis of Striga hermonthica resistance in pearl millet (Pennisetum glaucum [L.] R. Br.). J Crop Improv. 2022. https://doi.org/10.1080/15427528.2022.2156960.
Runo S, Alakonya A, Machuka J, Sinha N. RNA interference as a resistance mechanism against crop parasites in Africa: a ‘Trojan horse’ approach. Pest Manag Sci. 2011;67:129–36.
Samejima H, Sugimoto Y. Recent research progress in combatting root parasitic weeds. Biotechnol Biotechnol Equip. 2018;32(2):221–40.
Samejima H, Babiker AG, Takikawa H, Sasaki M, Sugimoto Y. Practicality of the suicidal germination approach for controlling Striga hermonthica. Pest Manag Sci. 2016;72(11):2035–42.
Savary S, Willocquet L, Pethybridge SJ, Esker P, McRoberts N, Nelson A. The global burden of pathogens and pests on major food crops. Nat Ecol Evol. 2019;3(3):430–9.
Schneeweiss GM, Palomeque T, Colwell AE, Weiss-Schneeweiss H. Chromosome numbers and karyotype evolution in holoparasitic Orobanche (Orobanchaceae) and related genera. Am J Bot. 2004;91:439–48.
Scholes JD, Press MC. Striga infestation of cereal crops–an unsolved problem in resource limited agriculture. Curr Opin Plant Biol. 2008;11(2):180–6.
Schroeder C, Onyango K’Oloo T, Nar Bahadur R, Jick NA, Parzies HK, Gemenet DC. Potentials of hybrid maize varieties for smallholder farmers in Kenya: a review based on swot analysis. Afr J Food Agric Nutr Dev. 2013;13:7562–82.
Sedlazeck FJ, Lee H, Darby CA, Schatz MC. Piercing the dark matter: bioinformatics of long-range sequencing and mapping. Nat Rev Genet. 2018;19(6):329–46.
Shayanowako AIT, Shimelis H, Laing MD. Mwadzingeni I Genetic diversity of maize genotypes with variable resistance to Striga asiatica Based on SSR markers. Cereal Res Commun. 2018;46(4):668–78.
Shayanowako AIT, Shimelis H, Laing MD, Mwadzingeni L. Striga resistance and compatibility of maize genotypes to a biocontrol agent, Fusarium oxysporum f.sp. Strigae. J Crop Improv. 2020;34(4):437–54.
Sibhatu B. Review on Striga weed management. Int J Life Sci Sci Res. 2016;2(2):110–20.
Silberg TR, Renner K, Schmitt Olabisi L, Richardson RB, Chimonyo VGP, Uriona-Maldonado M, Basso BB, Mwale C. Modeling smallholder agricultural systems to manage Striga in the semi-arid tropics. Agric Syst. 2021;187:103008.
Singh M, Nara U. Genetic insights in pearl millet breeding in the genomic era: challenges and prospects. Plant Biotechnol Rep. 2023;17(1):15–37.
Spallek T, Mutuku M, Shirasu K. The genus Striga: a witch profile. Mol Plant Pathol. 2013;14(9):861–9.
Stanley AE, Menkir A, Ifie B, Paterne AA, Unachukwu NN, Meseka S, Gedil M. Association analysis for resistance to Striga hermonthica in diverse tropical maize inbred lines. Sci Rep. 2021;11(1):24193.
Tesso T, Gutema Z, Deressa A, Ejeta G. An integrated Striga management option offers effective control of Striga in Ethiopia. In: Ejeta G, Gressel J, editors. Integrating new technologies for Striga control: towards ending the witch-hunt. WORLD SCIENTIFIC: Singapore; 2007. p. 199–212.
Thomas H, Howarth CJ. Five ways to stay green. J Exp Bot. 2000;51:329–37.
Upadhyaya HD, Reddy KN, Ahmed MI, Gowda CLL. Identification of gaps in pearl millet germplasm from East and Southern Africa conserved at the ICRISAT genebank. Plant Genet Resour. 2012;10(3):202–13.
Varshney RK, Tuberosa R. Genomics-assisted crop improvement: an overview. Genomics-assisted crop improvement: vol. 1: genomics approaches and platforms. Dordrecht: Springer; 2007. p. 1–12.
Varshney RK, Shi C, Thudi M, Mariac C, Wallace J, Qi P, Xu X. Pearl millet genome sequence provides a resource to improve agronomic traits in arid environments. Nat Biotechnol. 2017;35(10):969–76.
Wegmann K, Von Elert E, Harloff HJ, Stadler M. Tolerance and resistance to Orobanche. In Progress in Orobanche research. Proceedings of a Workshop on Orobanche, Eberhard-Karls-Universitat, Tubingen, Germany, 1991. p. 318–321.
Wilson J, Hess D, Hanna W, Kumar K, Gupta S. Pennisetum Glaucum Subsp. Monodii accessions with Striga resistance in West Africa. Crop Prot. 2004;23(9):865–70. https://doi.org/10.1016/j.cropro.2004.01.006.
Yacoubou AM, Zoumarou Wallis N, Menkir A, Zinsou VA, Onzo A, Garcia-Oliveira AL, Meseka S, Wende M, Gedil M, Agre P. Breeding maize (Zea mays) for Striga resistance: past, current and prospects in sub-Saharan Africa. Plant Breeding. 2021;140(2):195–210.
Yadav OP, Mitchell SE, Zamora A, Fulton TM, Kresovich S. Development of new simple sequence repeat markers for pearl millet. J SAT Agric Res. 2007;3(1):34.
Yadav OP, Rai KN. Genetic improvement of pearl millet in India. Agric Res. 2013;2:275–92.
Yadav OP, Gupta SK, Govindaraj M, Sharma R, Varshney RK, Srivastava RK, Mahala RS. Genetic gains in pearl millet in India: insights into historic breeding strategies and future perspective. Front Plant Sci. 2021;12:396.
Yasir AG, Abdalla HM. Introgression of Striga resistance genes into a Sudanese sorghum cultivar, Tabat, using marker assisted selection (MAS). Greener J Agric Sci. 2013;3(7):550–6.
Yoder JI, Scholes JD. Host plant resistance to parasitic weeds; recent progress and bottlenecks. Curr Opin Plant Biol. 2010;13(4):478–84.
Yoder JI, Gunathilake P, Wu B, Tomilova N, Tomilov AA. Engineering host resistance against parasitic weeds with RNA interference. Pest Manag Sci. 2009;65:460–6.
Yohannes T, Ngugi K, Ariga E, Abraha T, Yao N, Asami P, Ahonsi M. Genotypic variation for low Striga germination stimulation in Sorghum (Sorghum bicolor (L.) Moench”) landraces from eritrea. Am J Plant Sci. 2016;07(17):2470–82.
Yoneyama K, Awad AA, Xie X, Yoneyama K, Takeuchi Y. Strigolactones as germination stimulants for root parasitic plants. Plant Cell Physiol. 2010;51:1095–103.
Yoshida S, Shirasu K. Plants that attack plants: molecular elucidation of plant parasitism. Curr Opin Plant Biol. 2012;15(6):708–13.
Yoshida S, Ishida JK, Kamal NM, Ali AM, Namba S, Shirasu K. A full-length enriched cDNA library and expressed sequence tag analysis of the parasitic weed, Striga Hermonthica. BMC Plant Biol. 2010;10(1):1–10.
Zarafi A, Elzein A, Abdulkadir D, Beed F, Akinola O. Host range studies of Fusarium oxysporum f. sp. Strigae meant for the biological control of Striga hermonthica on maize and sorghum. Arch Phytopathol Plant Prot. 2015;48(1):1–9.
Zwanenburg B, Pospíšil T, Zeljković SĆ. Strigolactones: new plant hormones in action. Planta. 2016;243:1311–26.
Acknowledgements
The African Center for Crop Improvement (ACCI) is acknowledged for the administration work that facilitated the study of the first author.
Funding
The research was funded by the International Crops Research Institute for the Semi-Arid Tropics (ICRISAT) through the ‘Harnessing Opportunities for Productivity Enhancement (HOPE II) for Sorghum and Millets in sub-Saharan Africa’ project (OPP1198373), funded by the Bill & Melinda Gates Foundation (BMGF).
Author information
Authors and Affiliations
Contributions
All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Rouamba, A., Shimelis, H., Drabo, I. et al. Management of the Striga epidemics in pearl millet production: a review. CABI Agric Biosci 5, 11 (2024). https://doi.org/10.1186/s43170-023-00210-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43170-023-00210-1