Abstract
Background
Aquaculture is one of the critical sectors contributing to food and nutrition security, income and employment opportunities to millions of people, and is currently the fastest growing food-producing sector globally. With the global increase in aquaculture production, focus on biosecurity and fish health management is becoming increasingly important to address the risks and impacts of aquatic diseases. Within the framework of adaptive research, this study aimed at assessing the levels of awareness and preparedness of fish farmers in Western Kenya to meet fish health management and biosecurity requirements as important parameters determining the success of their farming activities.
Methods
A total of 504 fish farmers were interviewed using a pre-tested questionnaire generated by a computer-aided personal interview (CAPI). The data collected was summarized using descriptive statistics using SPSS version 25. The homogeneity of frequency data of all the parameters between the three counties was tested using a non-parametric Pearson Chi-Square test at α = 0.05.
Results
The key findings were that 76.1% of the fish farmers reported mortalities in their farms, with 2.3% reporting mortalities above 50% of the stocked fish, but with a majority (85.5%) reporting loss of up to 10%. In extrapolation, the total loss from the farms correlates with stagnation in aquaculture production in Kenya.
Conclusions
This study concludes that there is a paucity of knowledge on fish health management systems and biosecurity measures which presents a serious threat to aquaculture production in the studied counties and poses a great risk to trans-boundary live fish trade between Kenya and her neighbouring countries. Although fish farming is a promising area, it has had so many challenges among them high mortality rates. In China and other Asian countries, which are the world leaders in aquaculture production, they are making headway in fish health management and biosecurity. This study recommends the development of a coordinated awareness campaigns on fish health management and biosecurity measures to fish farmers in Kenya.
Similar content being viewed by others
Introduction
The increasing human population expected to add another 2 billion people by 2050 demands a parallel increase in food security. The importance of fish as a source of protein is exemplified by the 2030 Agenda of UN member states that sets aims for the contribution to fisheries and aquaculture towards food security and nutrition in the use of natural resources (FAO-SOFIA 2016). Aquaculture has been identified as one of the critical sectors contributing to food and nutrition security, income and employment opportunities to millions of people and is now the fastest growing food-producing sector globally (FAO 2020). In Kenya, aquaculture is mainly divided into mariculture, which is still at an infancy stage, and a more progressive freshwater aquaculture (Opiyo et al. 2018). The Government of Kenya has recognized this critical sector and has continued to invest towards intensification of aquaculture through different initiatives. For instance the economic stimulus programme (ESP) which resulted to a significant expansion from 4218 metric tonnes (MT) in 2006 to a peak of 24,096 in 2014 (Munguti et al. 2017; Obiero et al. 2019). As a result, freshwater aquaculture has been prioritized in some counties such as Kakamega, Siaya and Busia, with many fish farmers venturing into cage fish farming in the Winam Gulf of Lake Victoria (Munguti et al. 2014; Aura et al. 2018; Njiru et al. 2018). Intensive fish farming inevitably leads to increased prevalence of fish diseases and environmental contamination. Diseases are a direct hindrance to sustainability of aquaculture and can lead to reduced growth in the industry (for example, Norwegian Salmon industry 1980’s and today) or near total collapse (Chilean Salmonid Industry in 2007). In general, diseases have caused serious economic losses to finfish aquaculture around the world (Mustafa et al. 2001; Johnson et al. 2004; Sahoo et al. 2013; Monir et al. 2015). For example, in Brazil, fish losses due to mortalities have been estimated as leading to a total loss of US $84million annually (Tavares-Dias and Martins 2017).
The development of aquaculture in Africa has not been paralleled by measures that support its growth, namely disease surveillance, control and prevention, quality feed provision and water quality analysis and management practices.
Therefore, aquaculture has been associated with some challenges such as fish diseases and parasites (Shitote et al. 2013; Munguti et al. 2014; Ojwala et al. 2018; Opiyo et al. 2018). As a result, there should be adequate biosecurity measures to reduce economic losses through fish mortalities and unnecessary treatment costs (Bhujel 2014). Fish health management is a term used in aquaculture to describe management practices, which are designed to prevent fish diseases. A sustained and consistent practice of biosecurity is becoming an increasingly critical requirement for successful aquaculture (Noble and Summerfelt 1996; Browdy and Bratvold 1998; Timmons et al. 2002; Lee and O’Bryen 2003; Delabbio et al. 2005; Eissa et al. 2016). According to World Organization for Animal Health (OIE 2019), the term biosecurity can be defined as a set of management and physical measures designed to reduce the risk of introduction, establishment and spread of pathogenic agents to, and from and within an aquatic animal population. Therefore, unless governments are informed about the implications of fish diseases for the development of the fisheries and aquaculture sectors, and they act upon this information increasing support for fish health management, massive fish mortalities and economic losses may ensue (Akoll and Mwanja 2012). According to the recently held Aquatic Biosecurity Governance Workshop in Durban, South Africa in the year 2014, African states were urged to be proactive rather than reactive to a healthy aquaculture production that protects producers and the emerging sector from the risks and threats of aquatic pathogens and diseases (FAO 2018). Globally, the risks of pathogen introductions into aquaculture systems are on the rise (Kent 2000). This is due to different hosts being reared in new geographic areas, or by indigenous species being reared in a different environmental condition, i.e. the marine netpen. Examples of the former include Kudoa thyrsites (Myxozoa) and Hemobaphes disphaerocephalus (Copepoda) infections in Atlantic salmon (Salmo salar) reared in the Pacific Northwest, Ceratothoa gaudichaudii (Isopoda) infections in Atlantic salmon reared in Chile, Neoparamoeba (= Paramoeba) sp. (Sacromastigophora) from salmonids reared in Tasmania, and Stephanostomum tenue (Digenea) infections in rainbow trout (Oncorhynchus mykiss) reared in Atlantic Canada (Kent 2000). In addition, some previous studies have strongly recommended the need for baseline studies on fish emphasizing the health of farmed fish and health management practices to provide basic information for planning necessary interventions for fish health management in Kenya (Opiyo et al. 2018). Therefore, the aim of this study was to assess the fish health management and biosecurity practices in the Counties of Kakamega, Siaya and Busia, Kenya, where aquaculture has been prioritized for food security in the respective County Integrated Development Plans.
Materials and methods
Description of the study area
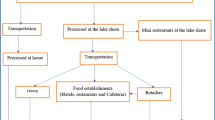
This study covered selected fish farms, cage farms and hatcheries in three counties in Western Kenya namely Kakamega, Siaya and Busia as shown in Fig. 1. A brief description of the characteristics of the three Counties is given below:
Kakamega County
Kakamega County is located in the Western part of Kenya and borders Vihiga County to the South, Siaya County to the West, Bungoma and Trans Nzoia Counties to the North and Nandi and Uasin-Gishu Counties to the East. The County covers an area of 3051.3 km2 and is the second populous county after Nairobi with the largest rural population. The altitude of the county ranges from 1240 to 2000 m above sea level. The southern part of the county is hilly and is made up of rugged granites rising in places to 1950 m above sea level. There are also several hills in the county such as Misango, Imanga, Eregi, Butieri, Sikhokhochole, Mawe Tatu, Lirhanda, Kiming’ini among others. There are ten main rivers in the county namely; Nzoia, Yala, Lusumu, Isiukhu, Sasala, Viratsi, Kipkaren, Kamehero, Lukusitsi and Sivilie. The annual rainfall in the county ranges from 1280.1 mm to 2214.1 mm per year. The rainfall pattern is evenly distributed all year round with March and October receiving heavy rains while December and February receives light rains. The temperatures range from 18 °C to 29 °C. According to the 2019 Population and Housing Census, the County population was 1,867,579. The county has a population growth rate of 2.5% with a population projection of 2,132,318 by the end of the year 2019 (County Government of Kakamega 2018).
Siaya County
Siaya County is one of the six counties in Nyanza region. It has a land surface area of approximately 2530 km2 and water surface area of approximately 1005 km2. It borders Busia County to the North West, Vihiga and Kakamega counties to the North East, Kisumu County to the South East and Homa Bay County across the Winam Gulf to the South. The water surface area forms part of Lake Victoria. It approximately lies between latitude 0° 26′ S to 0° 18′ N and longitude 33° 58′ and 34° 33′ East. There are three major geomorphological areas in the county namely: Dissected Uplands, Moderate Lowlands and Yala Swamp. These areas have different relief, soils and land use patterns. The altitude of the County rises from 1140 m on the shores of Lake Victoria to 1400 m above sea level on the North. The County experiences a bi-modal rainfall, with long rains falling between March and June and short rains between September and December. The relief and the altitude influence its distribution and amount. Siaya County is drier in the southern part towards Bondo and Rarieda sub-counties and is wetter towards the higher altitudes in the northern part particularly Gem, Ugunja and Ugenya sub-counties. On the highlands, the rainfall ranges between 800 mm and 2000 mm while lower areas receive rainfall ranging between 800 and 1600 mm. Temperatures vary with altitude rising from 21 °C in the North East to about 22.5 °C along the shores of Lake Victoria while in the South, it ranges from mean minimum temperature of 16.3 °C and mean maximum temperature of 29.1 °C. Humidity is relatively high with mean evaporation being between 1800 mm to 2200 mm per annum within the County. The relative humidity ranges between 73% in the morning and 52% in the afternoon (County Government of Siaya 2018). According to the 2019 Population and Housing Census, the County population was 993,183.
Busia County
Busia County is situated in western Kenya and serves as the gateway for Kenya to the neighboring Uganda, with two border crossing points at Busia and Malaba towns. Agriculture, fishing and trade are the main economic activities in Busia County. Being the entry points between Kenya and Uganda, Busia and Malaba towns are thriving trade towns where livestock, agricultural products and manufactured goods are traded. Busia's climate is conducive for agriculture. Some of the crops that are grown within the county in small scale include maize, beans, sweet potatoes, millet, cassava, cotton, tobacco and sugar cane. Fishing is also a major economic activity in Busia, due to the nearby Lake Victoria that supports a huge population of fish including Nile Perch and Tilapia. Most parts of Busia County fall within the Lake Victoria Basin. The altitude varies from about 1130 m above sea level at the shores of Lake Victoria to a maximum of about 1500 m above sea level in the Samia and North Teso Hills. Busia County receives annual rainfall of between 760 and 2000 mm. 50% of the rainfall falls in the long rain season which is at its peak between late March and late May, while 25% falls during the short rains between August and October. The dry season with scattered rains falls from December to February. The temperatures for the whole county are more or less homogeneous. The annual mean maximum temperatures range between 26 °C and 30 °C while the mean minimum temperature range between 14 °C and 22 °C (County Government of Busia 2018). According to the 2019 Population and Housing Census, the County population was 893,681.
Description of the fish ponds and cages
The fish ponds are mainly earthen ponds of size 300m2 (Fig. 2). The cages are floating cages of sizes of 4 m*5 m*4 m (Fig. 3). The hatcheries are of different sizes with the most modern being Wakhungu hatchery in Busia County (an example showing Labed Cash hatchery, Kakamega County (Fig. 4)).
Sampling technique
A modified systematic random sampling technique was used for sample selection. The rationale for choosing this technique is its simplicity and it also gives assurance that the population is evenly sampled. Field plan was prepared and finalized after consultation with the stakeholders. There were nine, Focus Group Discussions (FGDs), with three in each county and five (5) Key Informant Interviews with the major stakeholders. Mixed-sex groups were used for the FGDs since the survey was not considered to include ‘gender sensitive’ topics. The FGDs were chosen in such a way to ensure they included a minimum of 30% female respondents.
Sample size determination
The sample of this research was calculated by using the formula of Yamane (1973) with 95% confidence level.
The calculation formula was as follows:
where n = sample size required; N = number of people in the population; e = allowable error (%).
For the three counties, the total population of 3,754,443, and the target sample size was approximately 400. Considering that not all the populace are fish farmers, this study included all active farmers, with active fish farms, cages and hatcheries. A total of 504 farmers from three counties spread over 11 sub counties were interviewed based on their consent to participate and availability at the time of the study (Table 1).
Data collection
A semi-structured questionnaire was used for data collection using computer aided personal interview (CAPI) and data stored in Open Data Kit (ODK). The questionnaire was used to collect information on fish health management, biosecurity measures, common fish diseases and parasites, fish mortality and mitigation measures. Prior to the main survey, the questionnaire was pretested through ten in-depth interviews with the smallholder fish farmers in the project areas with the aim of testing and validation of the various aspects of the survey including data collection instruments, methodology and field logistics.
Data analysis
The data collected was summarized using descriptive statistics using SPSS version 25. The number of valid cases for data analysis was 495. The homogeneity of frequency data of all the parameters among the three counties was tested using a non-parametric Pearson Chi-Square test at α = 0.05. To test for association between mortalities and fish health management practices including biosecurity, the reported practices were categorized into distinct groups and responses tallied and a Fisher’s exact test was performed using R programming by collapsing the practices into two: those who practices some form of fish health management and those who do not.
Results
Fish health management systems
From this study, 76.1%, (n = 243) of the fish farmers reported mortalities in their farms (Fig. 5a), with 2.3% reporting mortalities above 50% of the stocked fish (Fig. 5b), but with a majority 85.5% reporting loss of up to 10% (Fig. 5b). There was no homogeneity between the three counties in terms fish mortality levels (Pearson Chi-Square test = 94.536, p < 0.005). Although these numbers seem to be low, if one extrapolates the total loss from the farms, then one can know why aquaculture production is stagnating. For the average farmer with a fish pond of 300 m2, 10% loss in all the farms amounts to approximately 25MT for Western fish ponds annually, and if it is the cages, the loss is even higher. Interestingly, 66.7%, (n = 243) of the fish farmers have never seen a sick fish (Fig. 5c). However, some of the fish farmers (32.5%) seemed well aware that water quality management was critical to avoid stressing the fish, hence controlling fish diseases (Fig. 5d).
Biosecurity measures
Some management practices reported included choosing springs/streams as the source of water to the farms (49%), use of inlet screens (2%) to keep off unwanted organisms, use of liming (5%) to ensure the farms are well buffered hence moderate the pH fluctuations and regular water exchanges in fish ponds were reported. There was no homogeneity between the three counties in terms of biosecurity measures (Pearson Chi Square test = 106.170, p < 0.005). Only (< 1%) of the farms in the study use disinfection (Footbath) to prevent any possible introduction of pathogens into the system. A detailed analysis of the various biosecurity measures and health management practices per county is shown in Fig. 6. There were no significant differences in the practices between the counties. For example, there were homogeneity in the regular removal of dead fish (Pearson Chi-Square test = 0.52, p > 0.05), fertilizer and liming (Pearson Chi-Square test = 4.11, p > 0.05) and water exchanges (Pearson Chi-Square test = 5.99, p > 0.05). There was no significant association between fish mortalities and fish health management practices and biosecurity measures (Fishers exact test, p = 0.3348).
Showing the various biosecurity measures and fish health management practices in a Busia County, b Siaya County and c Kakamega counties. A: no knowledge on biosecurity, B: Regular removal of dead fish, C: Cleaning of the ponds, D: Monitoring of water quality, E: Prevention of surface runoff, F: Fertilization and liming, G: Screening at the inlet, H: Water exchanges, I: Use of quality feeds, J: Good stocking rates, K: Use of bird nets and L: Disinfection
Common fish diseases and parasites in the three counties
The most recognizable disease by most farmers was the fungal infections, and other minor diseases such as cysts on the skin. Most farmers reported loss of scales, swollen scale, abnormal swimming activities, fin rots, and gulping for oxygen as common symptoms. Most of the diseases reported occurred during the rainy/cold season. The most commonly sighted parasite by the farmers is the leech (Hirudinea). In the present study, 60% of farmers would report incidences of sickness in the farm to the fisheries officers. There was no homogeneity between the three counties in terms of common fish diseases and parasites (Pearson Chi Square test = 180.748, p < 0.005).
Discussion
This study revealed huge losses of fish through mortalities with most fish farmers not practicing biosecurity measures to prevent fish diseases and infections in the three counties probably due to a lack of awareness. It is widely accepted globally that many people have not given fish mortalities the attention it deserves as a fish welfare issue (Ellis et al. 2012). This was the first study focusing on groundtruthing of the fish health management and biosecurity measures in fish farms in Kenya.
Source of water for fish farming in Western Kenya
Springs are the most preferred source of water in Western counties of Kenya because farmers consider the need to site farms in areas with permanent sources of water (Nguka et al. 2017). Besides being considered as a permanent water source, springs also have the best water quality with little or no known pathogens infecting fish. High water quality and its maintenance is considered a major mitigation against stress which predispose fish to diseases. Shitote et al. (2013) also reported the same results in Western Kenya. Other farmers (20%) rely on water from rivers for the fish farming operations. On the contrary farmers from the Central Kenya have been reported to use permanent rivers at 61% followed by public piped untreated water at 27% (Wanja et al. 2020).
Common fish diseases and parasites in the three counties
It was noted generally that most fish farmers (76%) reported mortality in their hatcheries and farms with an overall loss of about 10%, but did not attribute the mortalities to fish diseases, hence seem to have accepted mortalities as being a normal occurrence. By extrapolation, for about 4500 fish farms in Kenya, then the total loss is 450MT, accounting for US $2.25Million in losses. Such high losses have been reported elsewhere. For example, in Brazil, a loss of up to US $84 million has been reported annually for about 16,100 fish farms (Tavares-Dias and Martins 2017). The most recognizable disease by most farmers was the fungal infections, and other minor diseases such as cysts on the skin. Most of the diseases reported occurred during the rainy/cold season. This is probably due to the fact that the two main fish species (Nile tilapia and African catfish) cultured in the region are warmwater species which thrive well in high temperatures, with deaths occurring at 12 °C (Ngugi et al. 2007). The most commonly sighted parasite by the farmers was the leech (Hirudinea). This finding is strikingly similar to that of Omasaki et al. (2013) in which leeches were also reported to be the common parasites infecting fish from the Counties of Kakamega, Kisii and Siaya.
Biosecurity measures
A neglible percentage (< 2%) of the studied farms, for instance in Wakhungu Hatcheries in Busia County, did the study find the use of disinfection to prevent any possible introduction of pathogens into the system. This is very low as compared to other studies. For example, Faruk et al. (2012) reported that 76.66% of the hatcheries in Bangladesh used disinfection of equipments as a biosecurity measure. This could be possibly due to a lack of awareness of importance. This challenge can have impacts which can be detrimental to the aquaculture sector, not only in the studied areas but also to the wider region given the transboundary trade in live fish. As indicated by Assefa and Abunna (2018), biosecurity measures are very critical in preventing entry of pathogens to farms.
With the global increase in aquaculture production, focus on biosecurity and fish health management is becoming increasingly important to meet the risks and impacts of aquatic diseases (Bondad-Reantaso et al. 2005). This is the first study focusing on fish health management and biosecurity measures in Kenya. The topic of fish health management, a significant input factor in modern fish farming seems to have undergone little scrutiny in the context of successful industry development in Sub-Saharan Africa (Børge 2018). The findings of this study on biosecurity are strikingly similar to the study in Uganda, which found a low level diseases knowledge and awareness, some basic biosecurity measures being carried out in hatchers, but very few or no basic biosecurity measures are implemented routinely in grow-out farms (Børge 2018). Aquaculture has been touted as a promising solution for food insecurity, poverty and malnutrition in Kenya (Ogello and Munguti 2016), and indeed the Government of Kenya continues to invest heavily towards the intensification of aquaculture. Unfortunately, in the recent times, some of the most infectious diseases which can cause serious losses in farmed fish, some up to 90% such as Tilapia Lake Virus (TiLV) (Matolla 2018), Infectious Pancreatic Necrosis (Mulei et al. 2018) and the Infectious Haematopoietic Necrosis Virus (IHNV) the causative agent of infectious haematopoietic necrosis, a disease of salmonid responsible for great economic losses (Mulei et al 2019) have all been reported infecting fish in Kenya and Uganda. It is important to note that the country’s aquaculture production has been reported to be in a short fall of the required demand, hence imports such as those from China, and for us to have a true expansion in production, adequate fish health management is critical (Børge 2018). There are existing technologies in aquaculture including those of fish health management and biosecurity, however, there are gaps in technical skills, which hinder the uptake of those technologies and best management practices (Obiero et al. 2019). The most successful countries in terms of aquaculture production mainly in Asia and led by China have adequate fish health management systems and biosecurity measures (Mohammed and Subasinghe 2017). For example, with the onset of Tilapia Lake Virus (TiLV) outbreaks in several Asian countries, WorldFish in collaboration with Bangladesh’s Department of Fisheries, has developed a program to improve biosecurity in the tilapia industry throughout the country (Mohammed and Subasinghe 2017).
Conclusions
Western Kenya is one of the leading regions for aquaculture production in Kenya. This study concludes that there is a paucity of knowledge on fish health management systems and biosecurity measures, which presents a serious threat to aquaculture production in the studied counties and poses a great risk to trans-boundary live fish trade between Kenya and her neighbouring countries. Although fish farming is a promising area, it has had so many challenges among them high mortality rates. In China and other Asian countries, which are the world leaders in aquaculture production, they are making headway in fish health management and biosecurity. Lessons can be learnt from these countries and under the concept of Blue Economy development, the Kenyan government can develop aquaculture. Therefore, it will require collaborative efforts between the relevant ministry, departments and research institutions to formulate a strategy for the effective development, awareness creation, and implementation of the best fish health management practices and aquaculture biosecurity plans for the country (Additional files 1, 2 and 3).
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on request.
References
Akoll P, Mwanja WW. Fish health status, research and management in East Africa: past and present. Afri J Aquat Sci. 2012;37(2):117–29. https://doi.org/10.2989/16085914.2012.694628.
Assefa A, Abunna F. Maintenance of fish health in aquaculture: review of epidemiological approaches for prevention and control of infectious disease of fish. Vet Med Int. 2018. https://doi.org/10.1155/2018/5432497.
Aura CM, Musa S, Yongo E, Okechi JK, Njiru JM, Ogari Z, et al. Integration of mapping and socio-economic status of cage culture: towards balancing lake-use and culture fisheries in Lake Victoria, Kenya. Aquac Res. 2018;49:532–45. https://doi.org/10.1111/are.13484.
Bhujel RC. Fish health management and biosecurity. In: Bhujel RC (Ed) A manual for Tilapia business management. 2014; https://doi.org/10.1079/9781780641362.0136
Bondad-Reantaso MG, Subasinghe RP, Arthur JR, Ogawa K, Chinabut S, Adlard R, Tan Z, Shariff M. Disease and health management in Asian aquaculture. Rev Vet Parasitol. 2005;132(3–4):249–72. https://doi.org/10.1016/j.vetpar.2005.07.005.
Børge NF. Fish health management in Uganda. Tromsø: MSc Thesis. The Arctic University of Norway; 2018.
Browdy C, Bratvold D. Preliminary development of a biosecure production system. In: Moss S, editor. Proceedings of the U.S. marine shrimp farming program biosecurity workshop. Waimanolo: Oceanic Institute; 1998.
County Government of Busia. Busia County Integrated Development Plan 2018–2022. http://repository.kippra.or.ke/handle/123456789/1200
County Government of Kakamega. Kakamega County Integrated Development Plan 2018–2022. http://repository.kippra.or.ke/handle/123456789/1132
County Government of Siaya. Siaya County Integrated Development Plan 2018–2022. https://siaya.go.ke/siaya-county-integrated-development-plan-2018-2022/
Delabbio J, Murphy B, Johnson GR, McMullin SL. An assessment of biosecurity utilization in the recirculation sector of finfish aqua-culture in the United States and Canada. Aquac. 2004;242:165–79.
Delabbio J, Johnson G, Murphy B, Hallerman E, Woart A, Mcmullin S. Fish disease and biosecurity: attitudes, beliefs, and perceptions of managers and owners of commercial finfish recirculating facilities in the United States and Canada. J Aquat Anim Health. 2005;17:153–9. https://doi.org/10.1577/H04-005.1.
Eissa AE, Moustafa M, Abumhara A, Hosni M. Future prospects of biosecurity strategies in Egyptian fish farms. J Fish Aquat Sci. 2016;11(2):100–7.
Ellis T, Berrill I, Lines J, Turnbull JF, Knowles TG. Mortality and fish welfare. Fish Physiol Biochem. 2012;38:189–99. https://doi.org/10.1007/s10695-011-9547-3.
FAO. Development of a Regional Aquatic Biosecurity Strategy for the Southern African Development Community (SADC) FAO Fisheries and Aquaculture Circular No. C1149. Rome. 344 pp. 2018
FAO. The State of World Fisheries and Aquaculture 2020. Sustainability in action. Rome. 2020https://doi.org/10.4060/ca9229en
FAO-SOFIA. The state of World Fishereis and Aquaculture 2016. Fisheries development; small-scale fisheries; inland fisheries; nutrition; food security; fishery production; fishery byproducts. FAO, Rome, Italy. 2016; 204 pp.
Faruk MAR, Mony SFA, Hasan MM. Status of Biosecurity and Health Management in fish hatcheries. IRJALS. 2012;1(5):15–26.
Johnson SC, Treasurer JW, Bravo S, Nagasawa K, Kabata Z. A review of the impact of parasitic copepods on marine aquaculture. Zool Stud. 2004;43:229–43.
Kent ML. Marine netpen farming leads to infections with some unusual parasites. Int J Parasitol. 2000;30:321–6.
Lee CS, O’Bryen JP. Biosecurity in aqua-culture production systems: exclusion of pathogens and other desirables. Baton Rouge: World Aquaculture Society; 2003.
Matolla GK. Looming threat from disease: a wake up call for Kenyan tilapia fish production. Policy Brief. http://www.kilimo.go.ke/wp-content/uploads/2018/12/Geraldine-Matolla-Policy-Brief.pdf. 2018; Accessed 21 Jan 2021.
Mohamed DMS and Subasinghe R. Basic biosecurity manual for tilapia hatchery technicians in Bangladesh. Penang, Malaysia: CGIAR Research Program on Fish Agri-Food Systems. Manual: 2017; FISH-2017-10.
Monir S, Bagum N, Rahman S, Ashaf-Ud-Doulah M, Bhadra A, Borty SC. Parasitic diseases and estimation of loss due to infestation of parasites in Indian major carp culture ponds in Bangladesh. Int J Fish Aquat Stud. 2015;2:118–22.
Mulei IR, Nyaga PN, Mbuthia PG, Waruiru RM, Njagi LW, Mwihia EW, Gamil AAA, Evensen Ø, Mutoloki S. Infectious pancreatic necrosis virus isolated from farmed rainbow trout and tilapia in Kenya is identical to European isolates. J Fish Dis. 2018. https://doi.org/10.1111/jfd.12807.
Mulei IR, Nyaga PN, Mbuthia PG, Waruiru RM, Xu C, Evensen Ø, Mutoloki S. First detection and isolation of infectious haematopoietic necrosis virus from farmed rainbow trout in Nyeri County. Kenya J Fish Dis. 2019;5:751–8. https://doi.org/10.1111/jfd.12979.
Munguti J, Ogello E, Jeong-Dae K. An overview of Kenyan aquaculture: current status, challenges, and opportunities for future development. Fish Aquat Sci. 2014;17:1–11. https://doi.org/10.5657/FAS.2014.0001.
Munguti JM, Obiero KO, Orina PS, Mwaluma J, Mirera D, Ochiewo J, Kairo J, Njiru MJ, editors. State of aquaculture in Kenya. 1st ed. WestLink Services Limited: Nairobi; 2017.
Mustafa A, Rankaduwa W, Campbell P. Estimating the cost of sea lice to salmon aquaculture in eastern Canada. Can Vet J. 2001;41:54–6.
Ngugi CC, Bowman JR and Omolo BO. A new guide to fish farming in Kenya. 2007; 100pp. ISBN 978-0-9798658-0-0
Nguka G, Shitote Z, Wakhungu J, China S. Effect of fish farming on household food security in western Kenya. Afric J Food Agric Nutr Dev. 2017;17(01):11657–72. https://doi.org/10.18697/ajfand.77.15965.
Njiru JM, Aura CM, Okechi JK. Cage fish culture in Lake Victoria: a boon or a disaster in waiting? Fish Manag Ecol. 2018. https://doi.org/10.1111/fme.12283.
Noble A, Summerfelt ST. Diseases encountered in rainbow trout cultured in recirculating systems. Annu Rev Fish Dis. 1996;6:65–92.
Obiero KO, Waidbacher H, Nyawanda BO, Munguti JM, Manyala JO, Kaunda-Arara B. Predicting uptake of aquaculture technologies among smallholder fish farmers in Kenya. Aquac Int. 2019;27:1689–707.
Ogello EO, Munguti JM. Aquaculture: a promising solution for food insecurity, poverty and malnutrition in Kenya. Afri J Food, Agric, Nutr and Dev. 2016;16(4):1131–11350.
OIE. Aquatic Animal Health Code.2019; https://www.oie.int/index.php?id=171&L=0&htmfile=glossaire.htm. Accessed 31 Jan 2021.
Ojwala RA, Otachi EO, Kitaka NK. Effect of water quality on the parasite assemblages infecting Nile tilapia in selected fish farms in Nakuru County. Kenya Parasitol Res. 2018. https://doi.org/10.1007/s00436-018-6042-0.
Omasaki SK, Charo-Karisa H and Kosgey IS. Fish production practices of smallholder farmers in western Kenya. Livestock Res Rural Dev 2013; 25. http://www.lrrd.org/lrrd25/3/omas25052.htm. Accessed 19 Jan 2021.
Opiyo MA, Marijani E, Muendo P, Odede R, Leschen W, Charo-Karisa H. A review of aquaculture production and health management practices of farmed fish in Kenya. Int J Vet Sci Med. 2018;6(2):141–8. https://doi.org/10.1016/j.ijvsm.2018.07.001.
Sahoo PK, Mohanty J, Garnayak SK, Mohanty BR, Kar B, Prasanth H, Jena JK. Estimation of loss due to argulosis in carp culture ponds in India. Indian J Fish. 2013;60:99–102.
Shitote Z, Wakhungu J, China S. Challenges facing fish farming development in Western Kenya. Greener J Agric Sci. 2013;3(5):305–11. https://doi.org/10.15580/GJAS.2013.5.012213403.
Tavares-Dias M, Martins ML. An overall estimation of losses caused by diseases in the Brazilian fish farms. J Parasit Dis. 2017;41:913–8. https://doi.org/10.1007/s12639-017-0938-y.
Timmons MB, Ebeling JM, Wheaton FW, Summerfelt ST, Vinci BJ. Recirculating aquaculture systems. 2nd ed. Ithaca: Cornell University Press; 2002.
Wanja DW, Mbuthia PG, Waruiru RM, Mwadime JM, Bebora LC, Nyaga PN, Ngowi HA. Fish husbandry practices and water quality in Central Kenya: potential risk factors for fish mortality and infectious diseases. Vet Med Int. 2020. https://doi.org/10.1155/2020/6839354.
Yamane T. Statistics: an introductory analysis. New York: Harper and Row; 1973.
Acknowledgements
We gratefully acknowledge the County Fisheries Directors of Kakamega, Busia and Siaya Counties and their Fisheries Officers for assisting in the study. The authors thank the farmers who participated in this study. Views expressed herein do not necessarily reflect the official opinion of the funding organization.
Funding
This study was funded by KARLO- Kenya Climate Smart Agriculture Project. Grant Agreement Number GA02-4/1.
Author information
Authors and Affiliations
Contributions
DKM and ElO conceptualized the whole study and drafted the Manuscript. FA, ErO, KO, JA and CM participated in Data collection, data summarization and analysis and interpretation and edited the manuscript. JM: Supervised the study and edited the final copy. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The research was carried out under Ethics approval by the Egerton University Ethics Approval Committee.
Consent for publication
All authors have consented to the publication.
Competing interests
The authors declare that there are no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Fish health management questionnaire.
Additional file 2.
Inferential statistics.
Additional file 3.
Raw data file.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kyule-Muendo, D., Otachi, E., Awour, F. et al. Status of fish health management and biosecurity measures in fish farms, cages and hatcheries in Western Kenya. CABI Agric Biosci 3, 18 (2022). https://doi.org/10.1186/s43170-022-00086-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43170-022-00086-7