Abstract
Background
Acute pulmonary embolism (APE) is a serious illness. Identifying prognostic factors for APE may help in the management of those patients. This study’s objective was to evaluate the prognostic value of laboratory markers in predicting right ventricular dysfunction (RVD) and 30-day mortality in pulmonary embolism patients.
Methods
Eighty patients with APE were enrolled and followed up for 30 days. Detailed echocardiography was done to evaluate RVD. All patients were subjected to arterial blood gas analysis, complete blood count (CBC), plasma concentration of C-reactive protein (CRP), serum D-dimer level, and serum troponin I level, and the following ratio were calculated: neutrophil-to-lymphocytic ratio (NLR), platelet to lymphocytic ratio (PLR), red cell distribution width (RDW), mean platelet volume (MPV), and alveolar to arterial gradient.
Results
Our results analysis revealed significantly elevated levels of median NLR, PLR, CRP, D-dimer, and troponin in both the RVD and non-survivor groups (P value < 0.001). The blood markers that showed the highest predictive ability for right ventricular dysfunction (RVD) and 30-day mortality, as determined by receiver operating characteristic (ROC) analysis and logistic regression, were A-a O2 gradient, serum troponin, CRP, D-dimer, NLR, and PLR (P value < 0.001).
Conclusion
Evaluation of different serum markers including NLR, PLR, RDW, CRP, D-dimer, troponin, and A-a O2 gradient is a simple and available marker for predicting right ventricular dysfunction (RVD) and 30-day mortality in patients with APE.
Trial registration
ClinicalTrials.gov ID: NCT04237974.
Similar content being viewed by others
Introduction
Acute pulmonary embolism (APE) is a life-threatening disease affecting the cardiovascular and respiratory systems, which has a high rate of mortality [1, 2]. Assessing the potential adverse outcome for a patient may influence treatment choices [2,3,4].
Aside from clinical assessments and imaging abnormalities, additional biomarkers and indices can be employed to forecast the severity and prognosis of patients with APE. The biomarkers initially included serum troponin and brain natriuretic peptide. These biomarkers are employed to signify right ventricular dysfunction (RVD) and injury to the myocardium. Other metrics to consider are C-reactive protein (CRP), D-dimer, arterial blood gases (ABG) values, and complete blood count (CBC) parameters. However, these biomarkers are not extensively researched or used, even though they are easily accessible and cost-effective, especially in impoverished countries [5,6,7,8,9]. The use of laboratory tests for early risk stratification relies on the assumption that predictive biomarkers of disease severity might identify patients who are more likely to experience adverse consequences. This allows for timely recognition and intervention, ensuring that limited medical resources are allocated to those patients who would benefit the most.
It is recommended to use echocardiography as the first imaging tool to assess RVD and rapidly initiate fibrinolytic therapy [10, 11]. Nevertheless, the challenges related to reliance on operators and technical aspects have limited the application of this imaging technique for the evaluation of the right side of the heart [12].
We aimed in this study to conduct the ability of some of the laboratory biomarkers as predictors of right ventricular dysfunction and 30-day mortality in cases of acute PE.
Methods
We performed this study as a prospective observational cross-sectional study. This study was conducted in the Department of Chest Diseases and Tuberculosis at Assuit University Hospital. The Scientific Ethics Committee of the Assuit Faculty of Medicine, Assuit University, approved the study.
We included patients aged more than 18 years old who presented in either the Emergency Department or Respiratory Intensive Care Unit (RICU) during the period from January 2021 to November 2022 with clinical manifestations suggestive of massive or sub-massive APE. The diagnosis of pulmonary embolism (PE) relies on the patient’s clinical likelihood, laboratory tests, and Computed Tomography Pulmonary Angiography (CTPA), which is considered the most reliable method [13]. We excluded PE patients from the study if they had one or more of the following: hematological or coagulation disorders, active infectious disease, other cardiopulmonary disease, previous PE, taking immunosuppressive medications, taking antiplatelet and/or anticoagulant medications, blood transfusion within 3 months, chronic renal failure, advanced liver disease.
The sample size was calculated using Epi-info7. According to the results of the study [14], the percentage of right ventricular dysfunction was 61%. Based on this percentage and with confidence limits of 7%, the minimum sample needed for the study was estimated to be 80 patients.
All patients enrolled in the trial underwent a comprehensive assessment of their medical history, clinical examination, and standard laboratory tests. The results were determined based on the mortality rate within 30 days and categorized as either survivors or non-survivors. Upon admission, venous blood samples were promptly extracted from the patients under study and thereafter transported to the laboratory for analysis. The samples were examined to determine the CBC, CRP, serum D-dimer level, and serum troponin I level. The following ratios were calculated:
-
Neutrophil-to-lymphocytic ratio (NLR): it was calculated by dividing the absolute neutrophil count by the absolute lymphocytic count. The reference range is from 1 to 2. Less than 0.7 and more than 3 are considered pathological [15].
-
Platelet to lymphocytic ratio (PLR): it was calculated by dividing the absolute platelet count by the absolute lymphocytic count. The reference range is from 75 to 199 [16].
-
Red cell distribution width (RDW): its abnormal levels may be indicative of a range of pathological processes such as inflammatory stress. The normal value is from 11.5 to 14.5%.
-
Mean platelet volume (MPV): MPV is a simple marker of platelet activation, which is considered the first step of thrombosis. The normal range is from 8.5 to 11 fl.
Arterial blood samples were collected to determine the partial pressure of arterial oxygen (PaO2), arterial carbon dioxide (PaCO2), and the alveolar-arterial oxygen (A-a O2) gradient equation was calculated [17].
Transthoracic echocardiography was performed for all patients at presentation using a Philips HD 11 (Philips Ultrasound, Bothell, WA, USA) for assessment of RVD. Right ventricular basal and mid diameter of more than 4.2 cm and 3.5 cm respectively are considered to be dilated. Impaired RV systolic function detected by TAPSE (Tricuspid annular plane systolic excursion) < 1.6 cm, and Pulsed tissue Doppler peak velocity at the annulus (S' velocity) < 10 cm/s [18].
Statistical analysis
Data analysis was conducted by SPSS version 20. Numerical data was assessed for normality by the Shapiro–Wilk test and presented by mean and standard deviation or median and range. The independent Sample t test/Mann–Whitney U test was used to compare the mean/median difference between the two groups. Roc curve analysis and univariate logistic regression analysis were conducted to identify potential predictors for the occurrence of RVD or mortality in patients with pulmonary embolism. Significant variables were then included in a multivariate logistic regression analysis to calculate the adjusted odds ratio (AOR). The level of statistical significance was set at a P value of less than 0.05.
Results
The mean age of our patients was 51.43 ± 16.88, 50 (62.5%) were females and 30 (37.5%) were males. The most frequent clinical presentations for pulmonary embolism among studied patients were dyspnea (95.0%) followed by chest pain (52.5%), hemodynamic instability (40.0%), hemoptysis (31.3%), lower limb swelling and pain (15.0%), cough (12.5%), and syncope (8.8%). 50% of patients received anticoagulants, 41.2% received thrombolytics and 8.8% received catheter-directed therapy (CDT). 60 patients (75%) were survived. and the rest of them were not.
Patients were classified into two groups: no RVD group and RVD group according to Echocardiography. Table 1 shows the association between the outcome and RVD among studied patients. There was a statistically significant higher percentage of non-survivors among the RVD group compared to the no RVD group (45.2% vs 2.6% respectively), p value < 0.001.
RVD patients had significantly lower PaO2, SPO2, and higher A-a O2 gradient compared to non-RVD patients (p value 0.001).
As regards the CBC parameters, the RVD group showed significantly higher mean RDW (p = 0.008) and median NLR, PLR (p = < 0.001) compared to the non-RVD group. However, the median MPV did not differ significantly between both groups.
In addition, the RVD group had considerably higher median CRP, D dimer, and troponin levels compared to the non-RVD group (p < 0.001) (Table 2).
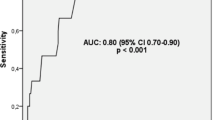
The most accurate serum markers able to predict RVD were A-a O2 gradient, serum troponin, CRP, D-dimer, NLR, RDW, and PLR in order as shown in Table 3 and Fig. 1.
Table 4 demonstrates the predictors attributed to the occurrence of RVD by univariate logistic regression analysis. These predictors included elevated A-a O2 gradient, increased RDW, elevated NLR, elevated PLR, elevated CRP, increased D-dimer, and elevated troponin levels. By multivariate logistic regression analysis, the only significant predictor was an increase in the A-a O2 gradient, with an odds ratio of 1.10 and a p value of 0.047.
Patients were classified according to 30-day mortality into two groups: survivors and non-survivors. Table 5 shows the association between ABG, serum markers, and mortality among studied patients. There was statistically significant lower mean PO2, SPO2, and higher mean A-a O2 gradient among the non-survivors. While, there was no statistically significant difference in mean RDW and mean MPV between survivors and non-survivors (16.57 ± 5.485 vs 17.59 ± 2.48, p value = 0.425), (9.01 ± 1.32 vs 9.68 ± 2.18, p value = 0.102) respectively. On the other hand, there was statistically significant higher median NLR, and PLR among non-survivors compared to survivors (9.15 vs 3.00, p value < 0.001), (270.00 vs 171.50, p value = 0.001) respectively. In addition, there was statistically significant higher median CRP, D dimer, and troponin among non-survivors, p value < 0.001.
Table 6 and Fig. 2 show the diagnostic ability of ABG and serum markers in the prediction of 30-day mortality, the most serum markers able to predict mortality were, D- dimer, A-a gradient, serum troponin, NLR, CRP, and PLR in order.
The significant predictors associated with 30-day mortality by univariate logistic regression analysis were increased A-a O2 gradient, increased NLR, increased CRP, increased D-dimer, and increased troponin level as displayed in Table 7, but none of them were significant by applying multivariate regression.
Discussion
Acute PE is a common clinical scenario and effective risk classification and death prediction methods are necessary to cope with different ranges of clinical care strategies for patients with APE.
Echocardiography and MSCT angiography may not always be accessible for assessing the prognosis of acute PE. In addition, patients may have chronic kidney disease, which is a contraindication for contrast administration.
This study aimed to identify the capability of particular laboratory indicators to anticipate right ventricular dysfunction and 30-day mortality in cases of APE.
We displayed that the RVD group had a statistically significantly higher median NLR and PLR, and mean red cell distribution width (RDW) than the non-RVD group, but the median mean platelet volume was not significantly different. In addition, the non-survivors had a statistically significantly higher median NLR, PLR, and median MPV, but no difference in mean RDW.
These findings were consistent with the studies conducted by Phan et al. and Jia et al. who found NLR and PLR were significantly higher in RVD patients than none RVD, and elevation of PLR and NLR suggests that acute pulmonary embolism is associated with a proinflammatory state [19, 20]. In et al. found that there was a higher mean RDW between both groups as in our study but differs from us in that he found that mean MPV was significantly higher in the RVD group [19]. Our study was not in agreement with Yardan et al. who reported that MPV was higher in the RVD group and stated that platelet activation is common in patients with acute PE and correlates with RV dysfunction [20]. These differences may be attributed to anticoagulant use, blood sampling timing, storage conditions, analytical techniques, calculation methods, accurate reference ranges, and diagnostic cut-offs, This lack of standardization makes data from different methods and analyzers hard to compare [21]. Ma et al. concurred with our study as they found statistically significant differences between survivors and non-survivors in NLR, and PLR and no difference between both groups in MPV [22].
The results of our study were in line with prior findings, which indicate that NLR is valuable for classifying risks in patients with venous thromboembolism [23]. PLR has been recognized as a novel indicator of systemic inflammation. It was found to have superior predictive value compared to platelet count or lymphocyte count alone in forecasting specific cardiovascular conditions [24]. Several studies also support our findings and found elevated NLR and PLR in non-survivors [25].
Our results exhibited statistically significant higher median C-reactive protein (CRP), D-dimer, and troponin in RVD and non-survivors groups. These results were in accordance with studies performed by Abul et al. and Keller et al. [7, 26]. These results contradicted the findings of Ohigashi et al., who observed no statistically significant disparity in the average levels of CRP, D-dimer, and troponin between the groups with and without RVD. They also concluded that BNP was a more dependable indicator of RVD and a complex course in patients with PE compared to other factors [27]. But Ohigashi et al. findings had many limitations, as they included only 50 patients retrospectively, and blood samples were withdrawn within 24 h of admission.
CRP levels are raised in cases of acute PE due to inflammatory reactions with subsequent pleural effusion development and hemorrhagic necrosis of pulmonary infarction [28].
In regard to the diagnostic accuracy of ABG and serum markers in the prediction of RVD and 30-day mortality, the most blood markers capable of predicting RVD were A-a O2 gradient, serum troponin, CRP, D-dimer, NLR, RDW, and PLR in order. In the study conducted by Jia et al., they found that NLR is more sensitive than troponin and D-dimer in predicting RVD with AUC = 0.803 which was not in agreement with our findings [29]. Ohigashi et al. declared that troponin, D-dimer, and CRP have high accuracy in predicting RVD with the area under the curve (0.703, 0.562, and 0.536 respectively) [27].
Roc curve of troponin in predicting RVD and 30-day mortality (at the cutoff point > 0.07 ng/ml). This was consistent with Henzler et al. who found the cutoff point of troponin 0.07 ng/ml with the area under the curve 0.70 [30]. Other studies found that the cutoff value of troponin in predicting RVD was 0.01 ng/ml which was different from our findings but agreed with us in that it has higher accuracy in predicting RVD [26, 31].
Limitations
There are some discrepancies between our findings and those from previous studies, and because of the different cut-off points, we suggest carrying out more research using a larger sample size of patients. Also, this will allow for a more thorough investigation into the causal relationship between various blood markers and the adverse outcomes caused by pulmonary embolism. Detection of RVD was assessed using TAPSE and pulsed tissue Doppler peak velocity at the TV annulus (S' velocity), and there are some other novel methods such as fractional area change and RV strain that have higher sensitivity in detecting RV dysfunction.
Conclusion
Evaluation of different serum markers including NLR, PLR, RDW, CRP, D-dimer, troponin, and A-a O2 gradient is a simple and available marker for predicting right ventricular dysfunction (RVD) and 30-day mortality in patients with APE.
Availability of data and materials
Data available on request.
Abbreviations
- A-a O2 :
-
Alveolar-arterial oxygen
- APE:
-
Acute pulmonary embolism
- AOR:
-
Adjusted odds ratio
- ABG:
-
Arterial blood gas analysis
- CBC:
-
Complete blood count
- CTPA:
-
Computed Tomography Pulmonary Angiography
- CRP:
-
C-reactive protein
- MPV:
-
Mean platelet volume
- NLR:
-
Neutrophil-to-lymphocytic ratio
- PaO2 :
-
Partial pressure of arterial oxygen
- PaCo2:
-
Partial arterial carbon dioxide
- PLR:
-
Platelet-to-lymphocytic ratio
- PE:
-
Pulmonary embolism
- RICU:
-
Respiratory Intensive Care Unit
- RDW:
-
Red cell distribution width
- RVD:
-
Right ventricular dysfunction
- ROC:
-
ROC curve analysis
- S' velocity:
-
Pulsed tissue Doppler peak velocity at the annulus
- TAPSE:
-
Tricuspid annular plane systolic excursion
References
Elias A, Mallett S, Daoud-Elias M, Poggi JN, Clarke M (2016) Prognostic models in acute pulmonary embolism: a systematic review and meta-analysis. BMJ Open. 6(4):e010324
de-Miguel-Diez J, López-de-Andrés A, Hernandez-Barrera V, Jimenez D, Monreal M, López-Herranz M et al (2021) The significance of heart failure in hospitalised patients with pulmonary embolism. A gender-specific analysis. Int J Clin Pract. 75(10):1–9
Jaff MR, McMurtry MS, Archer SL, Cushman M, Goldenberg N, Goldhaber SZ et al (2011) Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the american heart association. Circulation 123(16):1788–1830
Meyer G, Vicaut E, Danays T, Agnelli G, Becattini C, Beyer-Westendorf J et al (2014) Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 370(15):1402–1411
Lega JC, Lacasse Y, Lakhal L, Provencher S (2009) Natriuretic peptides and troponins in pulmonary embolism: a meta-analysis. Thorax 64(10):869–875
Soomro AY, Guerchicoff A, Nichols DJ, Suleman J, Dangas GD (2016) The current role and future prospects of D-dimer Biomarker. Eur Hear J - Cardiovasc Pharmacother 2(3):175–184
Abul Y, Karakurt S, Ozben B, Toprak A, Celikel T (2023) C-reactive protein in acute pulmonary embolism. J Investig Med 59(1):8–14
Liu J, Liu Y, Zhang F, Fu C, Ling Y, Fang P et al (2022) Short-term prognostic value of clinical data in hospitalized patients with intermediate-risk acute pulmonary embolism. BMC Cardiovasc Disord 22(1):1–14
Faghihi Langhroudi T, Borji Esfahani M, Khaheshi I, Naderian M, Zahedi Tajrishi F, Namazi MJ (2019) Correlation of hematologic indices with CT-pulmonary arterial obstruction index in patients with acute pulmonary emboli. Int J Cardiovasc Pract 4(3):89–93
Dabbouseh NM, Patel JJ, Bergl PA (2019) Role of echocardiography in managing acute pulmonary embolism. Heart 105(23):1785–1792
Bikdeli B, Lobo JL, Jiménez D, Green P, Fernández-Capitán C, Bura-Riviere A et al (2018) Early use of echocardiography in patients with acute pulmonary embolism: Findings from the RIETE registry. J Am Heart Assoc 7(17):1–7
Bing R, Chow V, Lau JK, Thomas L, Kritharides L, Ng ACC (2016) Prevalence of echocardiography use in patients hospitalized with confirmed acute pulmonary embolism: a Real-World observational multicenter study. PLoS ONE 11(12):1–13
Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, Harjola VP, ... , Zamorano JL (2019) The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): the Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur Respir J 54(3):1901647
Subramanian M, Ramadurai S, Arthur P, Gopalan S (2018) Hypoxia as an independent predictor of adverse outcomes in pulmonary embolism. Asian Cardiovasc Thorac Ann 26(1):38–43
Sarejloo S, Khanzadeh S, Hosseini S, Gargari MK, Lucke-Wold B, Mosalamiaghili S et al (2022) Role of the neutrophil to lymphocyte ratio in Guillain Barré syndrome: a systematic review and meta-analysis. Mediators Inflamm. 2022:3390831
Ni A (2016) Reference values of neutrophil-lymphocyte ratio, platelet-lymphocyte ratio and mean platelet volume in healthy adults in North Central Nigeria. J Blood Lymph 6(1):1–4
Abdelghany EA, Othman AM, Abdelfatah RA, Magdy M-E, Abd Elghany HS, Higazi MM (2019) Can alveolar—arterial oxygen gradient predict severity of pulmonary embolism? Egypt J Bronchol 13(2):273–279
Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K et al (2010) Guidelines for the echocardiographic assessment of the right heart in adults: a report from the american society of echocardiography. Endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and. J Am Soc Echocardiogr. 23(7):685–713
In E, Deveci F, Kaman D, Özdemir C, Sökücü SN, Kuluöztürk M et al (2015) The importance of mean platelet volume and red cell distribution width in acute pulmonary embolism. Acta Medica Mediterr 31(6):1209–1216
Yardan T, Meric M, Kati C, Celenk Y, Atici AG (2016) Mean platelet volume and mean platelet volume/platelet count ratio in risk stratification of pulmonary embolism. Med 52(2):110–115
Lippi G, Sanchis-Gomar F, Favaloro EJ (2020) Mean platelet volume in arterial and venous thrombotic disorders. J Lab Med 44(5):305–312
Ma Y, Mao Y, He X, Sun Y, Huang S, Qiu J (2016) The values of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in predicting 30 day mortality in patients with acute pulmonary embolism. BMC Cardiovasc Disord 16(1):1–6
Bakirci EM, Topcu S, Kalkan K, Tanboga IH, Borekci A, Sevimli S et al (2015) The role of the nonspecific inflammatory markers in determining the anatomic extent of venous thromboembolism. Clin Appl Thromb 21(2):181–185
Wang D, Yang JX, Cao DY, Wan XR, Feng FZ, Huang HF et al (2013) Preoperative neutrophil-lymphocyte and platelet-lymphocyte ratios as independent predictors of cervical stromal involvement in surgically treated endometrioid adenocarcinoma. Onco Targets Ther 6:211–216
Karataş MB, İpek G, Onuk T, Güngör B, Durmuş G, Çanga Y et al (2016) Assessment of prognostic value of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in patients with pulmonary embolism. Acta Cardiol Sin 32(3):313–320
Keller K, Beule J, Schulz A, Coldewey M, Dippold W, Balzer JO (2015) Cardiac troponin I for predicting right ventricular dysfunction and intermediate risk in patients with normotensive pulmonary embolism. Netherlands Hear J 23(1):55–61
Ohigashi H, Haraguchi G, Yoshikawa S, Sasaki T, Kimura S, Inagaki H et al (2010) Comparison of biomarkers for predicting disease severity and long-term respiratory prognosis in patients with acute pulmonary embolism. Int Heart J 51(6):416–420
Büyükşirin M, Anar C, Polat G, Karadeniz G (2021) Can the level of crp in acute pulmonary embolism determine early mortality? Turkish Thoracic Journal 22(1):4
Jia D, Liu F, Zhang Q, Zeng GQ, Li XL, Hou G (2018) Rapid on-site evaluation of routine biochemical parameters to predict right ventricular dysfunction in and the prognosis of patients with acute pulmonary embolism upon admission to the emergency room. J Clin Lab Anal 32(4):1–8
Henzler T, Roeger S, Meyer M, Schoepf UJ, Nance JW Jr, Haghi D et al (2012) Pulmonary embolism: CT signs and cardiac biomarkers for predicting right ventricular dysfunction. Eur Respir J. 39(4):919–26
Kucher N, Wallmann D, Carone A, Windecker S, Meier B, Hess OM (2003) Incremental prognostic value of troponin I and echocardiography in patients with acute pulmonary embolism. Eur Heart J 24(18):1651–1656
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
All the authors participated in the conception and design. AA collected the data and samples. AO, AF, and YA were responsible for the analysis and interpretation of data. KM and AH were responsible for drafting the article. AO and AF revised it critically for final approval of the version to be published. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics approval and consent to participate in the study were approved by the Local Ethics Committee of the Assiut University Hospital, the committee’s reference number IRB no: 17200409. Informed consent was obtained from all the participants before enrollment.
Consent for publication
All the authors approved the manuscript for publication. Identifying images or other personal or clinical details of participants is “not applicable.” Consent for publication from the participants is “not applicable.”
Competing of interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Omar, A.O.M., Ahmed, Y.A.Y., Elfadl, AE.A.A. et al. Prognostic value of laboratory markers in patients with acute pulmonary embolism. Egypt J Bronchol 18, 47 (2024). https://doi.org/10.1186/s43168-024-00293-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43168-024-00293-y