Abstract
Background
COVID-19 pneumonia and respiratory failure are the leading causes of death in COVID-19 patients. Prone positioning was hypothesized to improve oxygenation in ARDS patients and is being studied in COVID-19, but the current evidence is still unclear regarding survival and hospitalization. We aimed to investigate the effect of prone positioning on oxygenation in patients with COVID-19 pneumonia and ARDS and to examine the factors associated with better/worse outcomes.
Methods
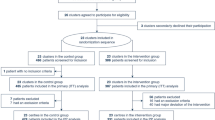
A retrospective record-based cohort study included all confirmed COVID-19 patients with pneumonia and ARDS who underwent prone positioning admitted to King Fahad Hospital, Medina, Saudi Arabia, during 2020–2021.
Results
This study included 75 cases (mean age 60.3 ± 15.7 year, 50 (66.7%) males), and all fulfilled the definition of ARDS. There was a significant improvement in oxygenation (PaO2 and PaO2/FIO2) following prone positioning (53.5 ± 6.8 vs. 60.4 ± 8.2 mmHg, p < 0.001 for PaO2 supine and prone and 120.3 ± 35 vs. 138 ± 40.2, p < 0.001 for PaO2/FIO2 supine and prone respectively). There was no significant difference in age, gender, smoking, or number of comorbidities between survivors and non-survivors. Survivors had significantly higher baseline PaO2 (p 0.018) and PF ratio (p 0.001) compared to non-survivors. They had also less severe inflammation and organ damage observed as significantly lower ferritin (p 0.001), D-dimer (p 0.026), aspartate aminotransferase (p 0.02), urea (p 0.032), creatinine (p 0.001), and higher platelet counts (p 0.001). Intubation and high-moderate comorbidity risk categories were associated with non-survival (p 0.001 and p 0.014, respectively).
Conclusion
Prone positioning is useful in the improvement of oxygenation in intubated and awake patients with COVID-19 pneumonia and ARDS. Intubation and high comorbidity risk categories were associated with non-survival.
Similar content being viewed by others
Background
COVID-19 is a major public health problem globally [1]. It is associated with pulmonary and extrapulmonary manifestations [2]. The development of hypoxemic respiratory failure and rapid deterioration increased the need for mechanical ventilation in many cases during the pandemic. Mortality was higher in older patients, those with comorbidities, and those with acute respiratory distress syndrome (ARDS) [3]. Prone positioning is an established technique to improve oxygenation in ARDS patients [4,5,6]. The same principle applies to COVID-19. Prone positioning improves gas exchange to dorsal lung regions through improving ventilation/perfusion ratio (V/Q), alveolar recruitment, decreasing total chest wall compliance, and reduction of nondependent lung mass [7]. It also improves the function of extrapulmonary organs as cardiac function and abdominal pressure [8]. Prone positioning in selected ARDS cases, if applied early, may improve survival [8]. Bellani et al. (2016) in their study involving intensive care units (ICUs) in 50 countries reported underutilization of prone positioning in patients with severe ARDS. They found that only 16.3% of patients with severe ARDS received prone positioning [9]. During the pandemic, the need for mechanical ventilation exceeded the number of available ICU beds in several countries [10]. In COVID-19, the mainstay of treatment of cases with respiratory failure is supportive care to improve oxygenation and lung recruitment [11]. It was proved to be useful in intubated [12] and non-intubated patients [13]. Prone positioning in COVID-19 patients may decrease the need for intubation and mechanical ventilation and decrease mortality [11]. Though ARDS guidelines up to 2021 did not include recommendations for the use of prone positioning in COVID-19 patients due to insufficient evidence [14], several studies reported the benefits of prone positioning in COVID-19 patients [15, 16]. Prone positioning was reported to improve oxygenation [17], but its effect on carbon dioxide is not consistent [18]. Previous studies reported several demographic factors such as age, gender, comorbidities, and laboratory data as ferritin, lactate dehydrogenase (LDH), and C-reactive protein (CRP) as determinants for morbidity and mortality [19,20,21]. However, the data are inconsistent in-between studies [22]. Evidence for the effect of prone positioning on survival is still insufficient [23].
Objectives
To examine the effect of prone positioning on oxygenation of COVID-19 patients with pneumonia and ARDS and to investigate the factors associated with inhospital mortality.
Methods
This is a retrospective record-based cohort study. We included all patients with COVID-19 pneumonia and ARDS who were admitted to King Fahad, Al-madinah Al-munawarrah, Medina, Saudi Arabia, from January 2020 to December 2021. All patients underwent prone positioning as part of the treatment protocol during 2020–2021.
Inclusion criteria
We included all patients with confirmed COVID-19 pneumonia and ARDS. A confirmed COVID-19 case is defined as a positive reverse transcriptase polymerase chain reaction assay for SARS-CoV-2 from nasopharyngeal swab samples [24]. Pneumonia diagnosis was based on the presence of clinical and/or radiologic signs of consolidation [25].
Exclusion criteria
These are suspected COVID-19, ARDS not due to COVID-19, mechanical ventilation due to other medical reasons, those on high-flow oxygen, or non-invasive mechanical ventilation. Patients with missing data are also excluded.
Prone positioning
Prone positioning was performed by the clinician according to the Saudi Ministry of Health guidelines for prone positioning in COVID-19 patients [26].
Indications and contraindications to prone positioning were assessed by the physician and not reported here. Assessment of the patient’s tolerance to prone positioning was performed within 5 min of the turn; failure of vital signs to return to baseline is considered intolerance. Prone positioning was discontinued in case of intolerance or in patients with worsening vital signs or oxygen desaturation after prone positioning (three cases). Prone positioning was done for an average of 12–16 h daily according to Saudi guidelines. For awake patients, it is 30–120 min in prone position and then 30–120 min in left lateral, right lateral, and upright positions. Continuous assessment of vital signs and SPO2 as well as sampling for arterial blood gases was done 20 min after prone positioning, and arterial blood gas samples were obtained at 1-h intervals [26].
Data collection
Patients’ files were reviewed, and we recorded demographic data, comorbidities, oxygenation parameters (PaO2, PF ratio), laboratory data results, and outcomes.
We revised the criteria for diagnosis [27, 28]. In addition, record PaO2, PaCO2, and PF ratio at baseline and 1 h after prone positioning on day 3 of prone positioning to avoid the effect of heterogeneity of data. PF ratio was calculated as PaO2/FIO2, and the severity of ARDS is considered as follows [mild (P/F > 200–300), moderate (P/F100–200), and severe (P/F < 100)] according to Berlin criteria [29].
We calculated the CHA2DS2-VASc comorbidity score (congestive heart failure, hypertension, age > 75, diabetes, prior stroke/transient ischemic attack, and vascular disease history). CHA2DS2-VASc comorbidity scores are categorized as low (0 score for men and 1 score for women), moderate-low (1 score for men and 2 score for women), and moderate-high (≥ 2 for men & ≥ 3 for women) [30].
Prone O2 responders were defined as those who had a 20-mmHg increase in PF ratio [15]. CO2 response is defined as the decrease of PaCO2 by 1 mmHg with prone positioning [31]. We also reviewed any reported complications to prone positioning.
Statistical analysis
Data was cleaned and coded. Analysis was performed using Statistical Package for Social Sciences (SPSS) for Windows version 26 (IBM Corp., Armonk, NY, USA). Categorical data were presented as numbers (percent), while continuous data was presented as mean ± SD for normally distributed data and median (IQR) for not normally distributed data. We used independent samples t-test and paired t-test for comparisons for normally distributed data, and Mann–Whitney U-test for non-distributed data. Association between categorical data was done using the chi-square test. Statistical significance was defined as p < 0.05.
Results
A total of patients 78 were reported, and in three of them, the prone position was discontinued due to desaturation. We included 75 cases and their mean age 60.3 ± 15.7, two-thirds are males 50 (66.7%), and 31 (41.3%) were smokers. All patients fulfilled definition of ARDS (Table 1). Comorbidities were prevalent in our patients 61 (81.3%), 28 (37.3%) had one comorbidity, and 33 (44%) had two or more comorbidities. Diabetes and hypertension were the most reported comorbidities (40% and 37.3%, respectively). The median CHASD-Vasc score was 2.
Out of 75 patients, 31 (41.3%) were intubated and mechanically ventilated, and 44 (58.7%) were spontaneously breathing awake. According to the definition of O2 and CO2 response, 31 (41.3%) were O2 responders, and 29 (38.7%) were CO2 responders.
No significant adverse events related to prone positioning were reported in the study population.
There was a significant improvement in mean PaO2, PaCO2, and PF ratio following prone positioning (paired samples t-test) (Table 2). Subgroup analysis showed oxygenation was significantly improved in moderate and severe ARDS categories (p 0.069, < 0.001, and 0.094 for PaO2 and p 0.068, < 0.001, and 0.008 for PF ratios in mild, moderate, and severe, respectively). While PaCO2 showed no significant change in all groups (p 0.464, 0.112, and 0.095 for mild, moderate, and severe ARDS, respectively). Both intubated and awake prone positioning had significant improvement in PaO and PF ratios (p < 0.001), while improvement in PaCO2 was only significant in the non-intubated group (p 0.006 for awake and 0.498 for intubated).
Most laboratory data showed abnormal levels, non-survivors had significantly higher ferritin, D-dimer, aspartate aminotransferase (AST), urea, and creatinine levels as well as significantly lower platelet counts (Table 3).
There was no significant statistical difference between the ARDS severity groups in terms of survival (p 0.382) as all patients in the mild ARDS survived 3 (100%), 39 of moderate ARDS (70.9%), and 10 of severe ARDS (58.8%) (Fig. 1). Also, there was no significant statistical difference in O2 response (p 0.521) or CO2 response (p 0.525) according to ARDS severity.
There was no significant statistical difference between survivors and non-survivors regarding age (mean age 64.61 ± 14.0 for survivors vs. 58.3 ± 16.2 for died, p 0.11). Non-intubation and low or low-moderate CHASD-Vasc categories were significantly associated with survival (Table 4).
Discussion
In this study, a total of 75 patients underwent prone positioning. Of them are 44 (58.7%) awake non-intubated patients and 31 (41.3%) intubated patients. We found that prone positioning significantly improves oxygenation (PaO2, PF ratio) in patients with COVID-19 pneumonia and ARDS (Table 2). Improved oxygenation was observed with either intubated or awake-prone positioning.
Improved oxygenation following prone positioning in mechanically ventilated patients has been reported in previous studies [15, 16, 32].
Chua et al. in their systematic review found low evidence for improved PF ratio in intubated COVID-19 patients who underwent prone positioning, with no evidence of improved survival [32]. Langer and colleagues reported improved oxygenation in mechanically ventilated patients [15].
The practice of prone positioning in awake ARDS was increased during the COVID-19 pandemic. Ehrmann and colleagues in their randomized controlled trial of COVID-19 reported that prone positioning improved oxygenation and reduced the need for intubation and incidence of treatment failure [16], while Fazzini et al. in their systematic review concluded that awake prone positioning improved oxygenation; however, it had an uncertain effect on intubation and survival [33]. Tompson et al. reported that prone positioning improved oxygenation (SPO2) in spontaneously breathing patients with COVID-19 ARDS (ref). Alhazzani et al. in a randomized clinical trial of COVID-19 patients reported that awake prone positioning had no significant effect on intubation [34].
The improved oxygenation may be attributed to improved V/Q mismatch by redistribution of flow from dorsal to ventral zones and alveolar recruitment [8, 15].
In this study, 31 (41.3%) showed oxygen response, and 29 (38.7%) showed CO2 response. The improved oxygenation is attributed to improved ventilation of dorsal lung regions, redistribution of edema from dorsal to ventral, and alveolar recruitment [18]. There is no fixed effect of prone position on carbon dioxide as it may increase, decrease, or stay constant according to changes in ventilation and perfusion [35].
In our study, no statistically significant difference was seen in age, sex, or smoking status between the oxygen responders and nonresponders or between CO2 responders and nonresponders. Oxygen responders had significantly higher baseline mean RBCs counts (4.5 ± 1.1 vs. 3.9 ± 1.0) and significantly lower post-prone positioning PaCO2 (43.5 ± 4.7 vs. 45.7 ± 10.3) compared to oxygen nonresponders.
Langer et al. reported that oxygen nonresponders had a more severe respiratory failure [15], while CO2 nonresponders were significantly older and had more comorbidities. No significant difference in outcomes was reported [15]. Changes in PaCO2 rather than PaO2 were related to lung recruitment [35].
The discrepancies between studies can be explained by the different demographic and clinical characteristics of patients in different studies. In our study, high mean RBCs number observed in O2 responders may contribute to better oxygen response.
In the current study, nearly two-thirds of patients 52 (69.3%) survived. Most survivors had awake prone positioning 37 (71.2%). Comorbidities were prevalent in our study, and diabetes and hypertension were the most common comorbidities reported (Table 1). No significant statistical difference was found between survivors and non-survivors regarding age, gender, number, and type of comorbidity. However, non-survivors had significantly higher comorbidity risk categories (p 0.014). High-moderate risk comorbidity scores were associated with death (Table 4).
These data are in line with previous studies that reported a high prevalence of comorbidities specifically diabetes and hypertension among COVID-19 in Saudi Arabia [19,20,21]. It is also reported that comorbidity risk scores (CHA2DS2-VASc) are more significant predictors than individual comorbidities (ref.).
Previous studies reported some demographic parameters such as age, gender, and comorbidity as predictors of mortality in COVID-19 patients [21].
A study investigated predictors of high risk among hospitalized COVID-19 patients in Saudi population reported older age, male gender, the presence of comorbidities, more severe lung infiltrate, high respiratory rate, abnormal blood urea nitrogen, and the need for mechanical ventilation [22].
The inconsistent results reported in various studies regarding the association between age, gender, and comorbidities with outcomes may be attributed to differences in sample sizes, study designs, and characteristics of the studied population in between studies.
In this study, non-survivors had significantly lower initial oxygenation parameters (PaO2, PF ratios) and platelet counts. They also had significantly higher baseline urea, creatinine, AST, ferritin, and D-dimer levels (Table 3) indicating more severe inflammation, lung injury, and organ dysfunction [36]. Higher D-dimer and low platelets in the dead patients in this study indicate more severe inflammation and increased incidence of thrombotic events due to activated coagulation pathway [37].
The same results were reported in previous studies that found high laboratory parameters among COVID-19 patients. These parameters were associated with the inflammatory process cytokine storm as well as sepsis and organ dysfunction [2].
High urea, creatinine, and LDH levels were reported among COVID-19 deaths in Saudi Arabia [22]. Some laboratory parameters such as procalcitonin, ferritin, D-dimer, C-reactive protein, and lymphocytes were reported as outcome predictors [38, 39].
The data vary across studies and no conclusion about which is the best predictor [22].
The broad variability of the range of laboratory data reported may explain the discrepancy of results in various studies.
The retrospective design of the study is a limitation. The results of this study are from a single-center, non-randomized sample, and the decision for prone positioning was made by the clinical management team, and standardization could not be controlled, and the observational nature of the study results could not be generalized; however, the results can be beneficial in designing randomized controlled trials and raising awareness of clinicians towards care of patients with comorbidities and abnormal laboratory data.
Conclusion
The results of this study indicate that prone positioning is useful in intubated and non-intubated patients with COVID-19 with ARDS. Intubation is associated with poor outcomes. Our results may help clinicians select patients who will benefit from prone positioning, and future randomized controlled studies including possible physiologic outcomes recording are warranted.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ARDS:
-
Acute respiratory distress syndrome
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- CHA2DS2-VASc:
-
Congestive heart failure, hypertension, age > 75, diabetes, prior stroke/transient ischemic attack, and vascular disease history
- COVID-19:
-
Coronavirus disease 2019
- FIO2:
-
Fraction of inspired oxygen
- ICUs:
-
Intensive care units
- L:
-
Liter
- mL:
-
Milliliter
- mmol:
-
Millimole
- ng:
-
Nanogram
- NLR:
-
Neutrophil-lymphocyte ratio
- P:
-
Prone
- PaO2:
-
Partial pressure of oxygen
- PaCO2:
-
Partial pressure of carbon dioxide
- PF:
-
PAO2/FIO2
- U:
-
Unit
- RBCs:
-
Red blood cells
- S:
-
Supine
- V/Q:
-
Ventilation/perfusion ratio
- WBCs:
-
White blood cells
References
WHO coronavirus (COVID-19) dashboard | WHO coronavirus (COVID-19) dashboard with vaccination data [Internet]. [cited 2022 Nov 19]. Available from: https://covid19.who.int/
Sobh E, Abuarrah E, Abdelsalam KG, Awad SS, Badawy MA, Fathelbab MA, et al. (2020 ) Novel coronavirus disease 2019 (COVID-19) non-respiratory involvement. Egypt J Bronchol 14(1):1–6. [cited 2022 Oct 8]. Available from: https://doi.org/10.1186/s43168-020-00030-1
Schmidt M, Hajage D, Demoule A, Pham T, Combes A, Dres M, et al. (2021) Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med 47(1):60–73. [cited 2022 Oct 28]. Available from: https://doi.org/10.1007/s00134-020-06294-x
Langer M, Mascheroni D, Marcolin R, Gattinoni L. (1988) The prone position in ARDS patients. A clinical study. Chest 94(1):103–7. [cited 2022 Oct 28]. Available from: http://journal.chestnet.org/article/S0012369216399159/fulltext
Bone RC. (1993 ) The ARDS lung: new insights from computed tomography. JAMA 269(16):2134–5. [cited 2022 Oct 28]. Available from: https://jamanetwork.com/journals/jama/fullarticle/405683
Guérin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T et al (2013) Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 368(23):2159–2168
Gattinoni L, Camporota L, Marini JJ. (2022) Prone position and COVID-19: mechanisms and effects∗. Crit Care Med 50(5):873–5. [Cited 2022 Oct 28]. Available from: https://journals.lww.com/ccmjournal/Fulltext/2022/05000/Prone_Position_and_COVID_19__Mechanisms_and.16.aspx
Scholten EL, Beitler JR, Kim Prisk; G, Malhotra A. Treatment of ARDS with prone positioning (2017) cited 2022 Oct 28. Available from: https://doi.org/10.1016/j.chest.2016.06.032
Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al. (2016 ) Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 315(8):788–800. [cited 2022 Oct 28]. Available from: https://jamanetwork.com/journals/jama/fullarticle/2492877
Douin DJ, Ward MJ, Lindsell CJ, Howell MP, Hough CL, Exline MC et al (2021 ) ICU bed utilization during the coronavirus disease 2019 pandemic in a multistate analysis—March to June 2020. Crit Care Explor 3(3):e0361. https://doi.org/10.1097/CCE.0000000000000361
Haleeqa MA, Alshamsi I, al Habib A, Noshi M, Abdullah S, Kamour A et al (2020) Optimizing supportive care in COVID-19 patients: a multidisciplinary approach. J Multidiscip Healthc 13:877–80. https://doi.org/10.2147/JMDH.S264168
Alhazzani W, Møller MH, Arabi YM, Loeb M, Gong MN, Fan E et al (2020) Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Crit Care Med E440–69. https://doi.org/10.1007/s00134-020-06022-5
Caputo ND, Strayer RJ, Levitan R. (2020) Early self‐proning in awake, non‐intubated patients in the emergency department: a single ED’s experience during the COVID‐19 pandemic. Acad Emerg Med 27(5):375. [cited 2022 Oct 28]. Available from: /pmc/articles/PMC7264594/
Tasaka S, Ohshimo S, Takeuchi M, Yasuda H, Ichikado K, Tsushima K, et al. (2022) ARDS Clinical Practice Guideline 2021. J Intensive Care 10(1):32. Available from: https://doi.org/10.1186/s40560-022-00615-6
Langer T, Brioni M, Guzzardella A, Carlesso E, Cabrini L, Castelli G et al (2021) Prone position in intubated, mechanically ventilated patients with COVID-19: a multi-centric study of more than 1000 patients. Crit Care. 25(1):1–11. https://doi.org/10.1186/s13054-021-03552-2. Cited 2022 Oct 28
Ehrmann S, Li J, Ibarra-Estrada M, Perez Y, Pavlov I, McNicholas B, et al. (2021 ) Awake prone positioning for COVID-19 acute hypoxaemic respiratory failure: a randomised, controlled, multinational, open-label meta-trial. Lancet Respir Med 9(12):1387–95. [cited 2022 Oct 28]. Available from: http://www.thelancet.com/article/S2213260021003568/fulltext
Scholten EL, Beitler JR, Prisk GK, Malhotra A (2017) Treatment of ARDS with prone positioning. Chest 151(1):215–224
Gattinoni L, Brusatori S, D’Albo R, Maj R, Velati M, Zinnato C et al (2023) Prone position: how understanding and clinical application of a technique progress with time. Anesthesiol Perioperative Sci 1(1):3. https://doi.org/10.1007/s44254-022-00002-2
Alsayer RM, Alsharif HM, Al Baadani AM, Kalam KA (2021) Clinical and epidemiological characteristics of COVID-19 mortality in Saudi Arabia. Saudi Med J 42(10):1083–1094
Alothaid H, Alshehri MA, Yusuf AO, Alzahrani ME, McDaniel J, Alamri S et al (2022) Sociodemographic predictors of confirmed COVID-19 mortality and hospitalization among patients in Saudi Arabia: analyzing a national COVID-19 database. J Infect Public Health 15(6):615–620
Surrati AMQ, Sobh E, Mansuri FA, Bokhari AA, Haroon SM, Alewi NM (2023) Sociodemographic and clinical characteristics of early COVID-19 deaths in Almadinah Almonawarah, Saudi Arabia: an analytical cross-sectional study. Pak J Med Sci 39(3):704–709
Ansari KA, Alwazzeh MJ, Alkuwaiti FA, Farooqi FA, Al Khathlan N, Almutawah H et al (2022) Early determinants of mortality in hospitalized COVID-19 patients in the Eastern Province of Saudi Arabia. Int J Gen Med 15:1689–1701
Grasselli G, Calfee CS, Camporota L, Poole D, Amato MBP, Antonelli M et al (2023) ESICM guidelines on acute respiratory distress syndrome: definition, phenotyping and respiratory support strategies. Intensive Care Med 49(7):727–759
Coronavirus disease 19 (COVID-19) guidelines (2020) [cited 2023 Oct 31]. available from https://covid19.cdc.gov.sa/wp-content/uploads/2023/04/V3.1COVID-19-Coronavirus-Disease-Guidelinesfinal-editionApr4-en.pdf
Kaysin A, Viera AJ. (2016) Community-acquired pneumonia in adults: diagnosis and management [Internet]. 94. Available from: www.aafp.org/afp.
Use of prone positioning in adult with COVID-19 respiratory failure [Internet]. [cited 2022 Oct 28]. Available from: https://www.moh.gov.sa/Ministry/MediaCenter/Publications/Documents/Prone-position-guidelines.pdf
Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. (2012) Acute respiratory distress syndrome: the Berlin definition. JAMA 307(23):2526–33. [cited 2022 Oct 28]. Available from: https://jamanetwork.com/journals/jama/fullarticle/1160659
Fan E, Brodie D, Slutsky AS. (2018) Acute respiratory distress syndrome: advances in diagnosis and treatment. JAMA 319(7):698–710. [cited 2022 Oct 28]. Available from: https://jamanetwork.com/journals/jama/fullarticle/2673154
Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. (2012) Acute respiratory distress syndrome: the Berlin definition. JAMA 307(23):2526–33. [cited 2022 Oct 29]. Available from: https://jamanetwork.com/journals/jama/fullarticle/1160659
Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HJGM, Andresen D, et al. (2010) Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on atrial fibrillation. Chest. 137(2):263–72. [cited 2022 Oct 28]. Available from: http://journal.chestnet.org/article/S0012369210600670/fulltext
Lemasson S, Ayzac L, Girard R, Gaillard S, Pavaday K, Guérin C (2006) Does gas exchange response to prone position predict mortality in hypoxemic acute respiratory failure? Intensive Care Med 32(12):1987–1993
Chua EX, Wong ZZ, Hasan MS, Atan R, Yunos NM, Yip HW et al (2022) Prone ventilation in intubated COVID-19 patients: a systematic review and meta-analysis. Braz J Anesthesiol 72(6):780–789. https://doi.org/10.1016/j.bjane.2022.06.007
Fazzini B, Page A, Pearse R, Puthucheary Z (2022) Prone positioning for non-intubated spontaneously breathing patients with acute hypoxaemic respiratory failure: a systematic review and meta-analysis. Bri J Anaesth 128:352–62
Alhazzani W, Parhar KKS, Weatherald J, Duhailib Z al, Alshahrani M, Al-Fares A, et al. (2022) Effect of awake prone positioning on endotracheal intubation in patients with COVID-19 and acute respiratory failure: a randomized clinical trial. JAMA 327(21):2104–13. [cited 2022 Oct 28]. Available from: https://jamanetwork.com/journals/jama/fullarticle/2792506
Protti A, Chiumello D, Cressoni M, Carlesso E, Mietto C, Berto V et al (2009) Relationship between gas exchange response to prone position and lung recruitability during acute respiratory failure. Intensive Care Med 35(6):1011–1017
Zhao W, Li H, Li J, Xu B, Xu J (2022) The mechanism of multiple organ dysfunction syndrome in patients with COVID-19. J Med Virol. 94:1886–92
Lehmann A, Prosch H, Zehetmayer S, Gysan MR, Bernitzky D, Vonbank K et al (2021) Impact of persistent D-dimer elevation following recovery from COVID-19. PLoS One 16(10):e0258351. https://doi.org/10.1371/journal.pone.0258351
Rai D, Ranjan A, H A, Pandey S ( 2021) Clinical and laboratory predictors of mortality in COVID-19 infection: a retrospective observational study in a tertiary care hospital of eastern India. Cureus 2;13(9):e17660. https://doi.org/10.7759/cureus.17660
Gopaul CD, Ventour D, Thomas D (2022) Laboratory predictors for COVID-19 intensive care unit admissions in Trinidad and Tobago. Dial Health 1:1
Acknowledgements
Not applicable
Funding
This study is not funded.
Author information
Authors and Affiliations
Contributions
All authors participated in the conception and design of the study, ZA, AI, AA, YAI, GA, DAI, and MAS performed data collection and management. ZA obtained ethical approval. ES and AHA analyzed and interpreted the data. ES wrote the draft, and all authors participated in the literature review and writing and revised the manuscript. ES, ZA, and AHA critical revision. All authors read and approved the manuscript
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study has been approved by the Institutional Review Board (IRB), General Directorate of Health Affairs in Madinah, Saudi Arabia (Ref. 23–026).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Adawy, Z., Iskandarani, A., Alharbi, A. et al. Response to prone positioning in COVID-19 patients with acute respiratory distress syndrome: a retrospective observational study. Egypt J Bronchol 18, 10 (2024). https://doi.org/10.1186/s43168-024-00261-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43168-024-00261-6