Abstract
Background
Some evidence has provided that electrolyte disorders may be present upon presentation of patients with COVID-19 infection. We investigated serum sodium, potassium, calcium, magnesium, and phosphorus levels in large numbers of critically ill COVID-19 patients to identify its possible prognostic value in these patients.
Methods
This retrospective study included patients confirmed with COVID-19 infection admitted to critical care units of Zagazig University Hospital all over 1 year, from May 1, 2020, to April 30, 2021. We analyzed the data for possible correlations between serum electrolytes and patients’ outcomes.
Results
Among 600 patients included in the study with a mean age of 51.33 ± 16.5 years, 44.16% were mechanically ventilated, and 30.66% died during hospital admission. Serum sodium, potassium, phosphorus, magnesium, and calcium were 141.96 ± 5.4, 4.33 ± 0.66, 3.76 ± 1.26, 2.21 ± 0.52, and 8.55 ± 0.85 respectively, at admission to the ICU. Unfavorable admission course and mortality were significantly associated with high normal serum sodium, potassium, and phosphorus levels and a low normal calcium level.
Conclusion
Although mean serum sodium, potassium, calcium, magnesium, and phosphorus were within normal levels in patients with COVID-19 at presentation, serum sodium, potassium, and phosphorus were significantly higher in those with poor outcomes, whereas calcium was significantly lower in those with poor outcomes.
Similar content being viewed by others
Introduction
Coronaviruses are a type of non-dividing positive-sense RNA viruses pathogenic in mammals, including humans, and belong to the Coronaviridae family [1]. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the seventh type of coronavirus class of human infections [2]. In addition to the respiratory system, COVID-19 has been shown to affect the gastrointestinal (GI) tract, neurological system, cardiovascular system, and urogenital system, according to previous studies [3].
Recognizing and describing people who are more likely to develop severe and critical COVID-19 are crucial for implementing strategies to protect or selectively vaccinate them [4]. Reliable predictors of disease severity, management response, and patient’s outcomes have not been properly investigated. When these indicators are present, appropriate care and optimal treatment can be received, which is believed to significantly reduce the mortality rate of severe patients [5].
In COVID-19, lymphopenia and thrombocytopenia, increased serum ferritin, interleukin-6 (IL-6) and IL-10 levels, coagulation parameter abnormalities such as elevated D-dimer levels, cardiac and muscle injury parameters changes, and liver and renal function abnormalities were all linked to disease severity and a poor outcome [6].
In some COVID-19 studies, evidence has been provided that electrolyte disturbances may be present upon patients’ presentation, including potassium, sodium, calcium, and chloride abnormalities [7]. Such electrolyte imbalances have significant effects on patient care as well as the identification of potential pathophysiologic pathways for COVID-19, which may result in the development of novel treatment alternatives [8].
SARS-CoV-2 invades human cells through binding angiotensin-converting enzyme 2 (ACE2) on the cell membrane, the principal counter-regulatory mechanism for the main axis of the renin-angiotensin system (RAS), which is an essential player in the control of blood pressure and electrolyte balance, leading to increased reabsorption of sodium and water and thereafter increased blood pressure and excretion of potassium (K +). Additionally, some COVID-19 individuals experienced problems with their GI tracts including vomiting and diarrhea [9, 10]. Hypokalemia, especially if severe, worsens acute cardiac injury and respiratory failure caused by the acute respiratory distress syndrome (ARDS), both of which are significant consequences in COVID-19 patients, particularly those who have underlying cardiac or lung illness [8, 11].
IL-6, which is one of the most important cytokines implicated in COVID-19-induced pathology, causes electrolyte impairment by inducing the non-osmotic release of vasopressin [12]. It may be hypothesized that a decrease in serum sodium indicates a more advanced disease, as sodium is an important factor for regulating the expression of ACE2 [13].
Also, hypocalcemia is highly prevalent among COVID-19 patients, which could be due to disturbances in intestinal absorption, an imbalance in regulatory mechanisms involving parathyroid and vitamin D, or a direct result of SARS-CoV-2 infection [14]. Alternating calcium homeostasis within the cell might trigger the inflammatory pathways and elevate levels of IL-1b, IL-6, and TNF linked to lung cell damage and edema accumulation [15].
In the literature, few studies have investigated the change in one or more electrolytes in patients with COVID-19, but to our knowledge, no study addressed the electrolyte status of patients at the time of admission to the intensive care units (ICUs) and its effect on patients’ prognosis. In our study, we investigated serum sodium, potassium, calcium, magnesium, and phosphorus levels in large numbers of critically ill patients infected with COVID-19 to detect their possible prognostic value.
Patients and methods
In this retrospective observational cohort study, we included all adult patients above 18 years of age admitted to critical care units of the Isolation Hospital of Zagazig University with a confirmed diagnosis of COVID-19 infection in the period between May 1, 2020, and April 30, 2021, with complete medical records.
A positive result of the real-time PCR test from a nasopharyngeal swab for SARS-CoV-2 defined the confirmed cases. The management of COVID-19 patients was carried out in accordance with local Egyptian COVID-19 management protocol [16]. We excluded patients with incomplete medical records and those who had other causes of electrolyte disturbances, e.g., chronic organ dysfunction (e.g., hepatic or renal dysfunction), terminal cancer, immunodeficiency, and patients receiving diuretics.
A trained team of medical staff reviewed and collected the recorded demographic, epidemiological, clinical, and laboratory data in a standardized data collection form modified from medical records. These data included patient characteristics at admission: gender, age, special habits, medications, and comorbidities (hypertension, diabetes mellitus, hyperlipidemia, asthma, cardiac or liver disease, etc.). The time of hospital and ICU admission, the need for intubation and mechanical ventilation days, the length of hospital stay, the presence of acute kidney injury (AKI), and the patient’s outcome (discharge or mortality). Clinical manifestations like fever, respiratory symptoms, and gastrointestinal symptoms were also recorded. Full laboratory investigations: complete blood count (CBC), kidney function tests (KFTs), liver function tests (LFTs), prothrombin time (PT), partial thromboplastin time (PTT), international normalized ratio (INR), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), serum lactate dehydrogenase (LDH), S ferritin, D-dimer, and serum electrolytes at admission to the critical care unit (Na, K, Ca, Ph, Mg), and computed tomography of the chest (CT) interpretation. SPO2 monitoring, arterial blood gas analysis, PaO2/FiO2, serum osmolality, base excess (BE), and pH were also reported.
Serum electrolytes were measured on Roche Cobas 8000 systems (Roche Diagnostics, Switzerland) at Zagazig University Hospital Laboratories using dedicated reagents supplied by the manufacturer. Sodium and potassium were measured on ion-selective electrode (ISE) units, while calcium, phosphorous, and magnesium were measured spectrophotometrically on c702 units.
The reference values for electrolytes and minerals were adapted from reagents inserts of analyzer manufacturer and validated in-house as per the GAHAR national accreditation guidelines [17]. The reference ranges are summarized in Table 1.
The main outcome was either patient discharge or patient mortality (primary outcome), whereas the patients with an unfavorable course (secondary outcome) included those who needed mechanical ventilation (invasive or non-invasive) during the admission course and/or those with multi-organ failure.
Ethical approval and trial registration
Our study was approved by the Institutional Review Boards of Faculty of Medicine, Zagazig University, with the reference number ZU-IRB#: 6328–23-8–2020, and it was registered with ClinicalTrials.gov (NCT04539834) at (07/09/2020).
Data collection is retrospective and anonymous. Therefore, the Institutional Review Boards of the Affiliated Hospital of Zagazig University waived the need for informed consent. All methods in this study were carried out in accordance with relevant guidelines and regulations (the Declarations of Helsinki).
Statistical analysis
Data analysis was performed using the software SPSS (Statistical Package for the Social Sciences) version 28. Quantitative variables were described using their means and standard deviations. Categorical variables were described using their absolute frequencies and compared using the chi-square test, Fisher exact test, and Monte Carlo test when appropriate. For ordinal binary data, the chi-squared for the trend test was used. Kolmogorov–Smirnov (distribution type) and Levene (homogeneity of variances) were used to confirm assumptions for use in parametric tests. To compare quantitative data between the two groups, an independent sample t-test (for normally distributed data) was used. Spearman rank and Pearson correlation coefficient (for not normally distributed and normally distributed data, respectively) were used to assess the strength and direction of correlation between quantitative data. The level of statistical significance was set at P < 0.05. A highly significant difference was present if p ≤ 0.001.
Results
At the time of the study, 629 cases of COVID-19 were admitted to the critical care units of the Isolation Hospital of Zagazig University. Twenty-nine patients met the exclusion criteria and were not included in the study. The study included 600 patients; their ages ranged from 21 to 93 years, with males representing 55.7% of them. About 29.1% and 34% of patients had comorbid diabetes and hypertension, respectively, and 39.5% of the patients received antiplatelet therapy before admission. Two hundred sixty-five cases (44.16%) received mechanical ventilation, either non-invasive or invasive ventilation. Non-invasive mechanical ventilation was used for 261 cases (43.5%), with median non-invasive ventilator days 16 (1–33). Four cases passed the course from oxygen therapy to the invasive one directly without non-invasive ventilation. One hundred eighty-five cases (30.83%) were supported by invasive mechanical ventilation; all of them died except for one that was successfully weaned with a better outcome. The median invasive ventilator days were 6 (1–17). Invasive mechanical ventilation was significantly associated with death (Table 2).
Table 3 shows the laboratory data for the patients at the time of ICU admission, with serum sodium, potassium, phosphorus, magnesium, and calcium of 141.96 ± 5.4, 4.33 ± 0.66, 3.76 ± 1.26, 2.21 ± 0.52, and 8.55 ± 0.85, respectively.
Our results showed that there was a statistically significant negative correlation between serum sodium level and platelet count, CRP, ALT, and AST values. Additionally, there was a statistically significant positive correlation between serum sodium levels and total bilirubin and ferritin. Regarding serum potassium, we noticed a statistically significant negative correlation between serum potassium and absolute lymphocytes, platelet count, total protein, and albumin, and a significant positive correlation between serum potassium and all PTT, INR, creatinine, BUN, LDH, and AST. Moreover, there were significant correlations between phosphorus, calcium, and magnesium and different laboratory data of the patients, as shown in Table 4.
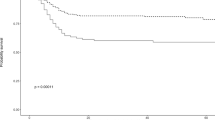
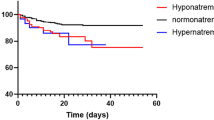
As can be seen from Tables 5 and 6, there was a significant relationship between the patients’ course and outcome with all sodium, potassium, calcium, and phosphorus levels. Unfavorable admission courses and patient mortality were significantly associated with higher serum sodium, potassium, phosphorus, and lower calcium levels.
Discussion
In our study, we addressed the electrolyte status of 600 patients admitted with COVID-19 and its relation to patient prognosis and different laboratory parameters. We found that mean serum sodium, potassium, calcium, magnesium, and phosphorus were within normal levels in patients with COVID-19. However, sodium, potassium, and phosphorus were significantly higher in those with poor outcomes, whereas calcium was significantly lower in those with poor outcomes.
Our study revealed that the mean sodium level of our patient was 141.96 ± 5.4 but the critically ill patients and those with mechanical ventilation had significantly higher sodium levels (143.59 ± 6.75). This is in concordance with a previous study that revealed that COVID-19 patients who were mechanically ventilated and those who were associated with a high mortality rate had significant hypernatremia [18].
Hyponatremia is common at the presentation of patients with COVID-19 due to inappropriate antidiuretic hormone secretion (SIADH). Lung pathologies with injury to the alveolar basement membrane, such as pneumonia, ARDS, and pulmonary malignancy, lead to indirect stimulation of IL-6 that induces the secretion of antidiuretic hormone (ADH) [19]. Hypernatremia is frequent in ICUs, with rates ranging from 4.3 to 15.8%. It is a warning sign and has been linked to longer hospital stays and a greater mortality rate. Because of saline infusions, the patient’s lack of autonomy, and their inability to drink when thirsty, the causes of ICU-related hypernatremia are generally believed to be iatrogenic [18]. Additionally, COVID-19 patients are at risk for hypovolemia because of anorexia, tachypnea, fever, and increased insensible water loss, which raises the risk of hypernatremia. Patients with hypernatremia frequently need more time in intensive care and have a higher mortality rate. As a result, the evaluation of ion levels such as sodium and potassium is an important prognostic indicator in COVID-19 patients [20]. An association between hypernatremia at admission and the need for mechanical ventilation and death has been recently discovered. It has been recommended that sodium levels be used as part of risk stratification strategies for disease severity [21].
Both hypokalemia and hyperkalemia have a poor prognostic value; critically ill patients in the medical ICU with abnormal serum potassium levels had a greater rate of ICU death than patients with normal potassium levels, according to a cohort study [22]. Increased serum potassium was found to be an independent predictor of mortality in individuals with severe community-acquired pneumonia in a prospective observational study [23]. Lower (3.5 mEq/L) and higher (4.5 mEq/L) serum potassium levels are both related to increased risk of mortality in patients with acute myocardial infarction, according to a systematic review and meta-analysis [24]. Patients with mean potassium values between 3.5 and 4.0 mmol/L had the lowest mortality, according to a large retrospective analysis [25].
In contrast to some reports in the literature, we noted that mean serum potassium was normal in admitted patients; moreover, high normal potassium level was associated with poor prognosis and higher mortality; this can be explained by first the presence of metabolic and respiratory acidosis with extra-cellular shifting of potassium. Second, the use of low molecular weight heparin in patients with COVID-19 is associated with higher potassium levels [26, 27]. Third, the role of Furin, a proprotein protease responsible for the higher infectivity of SARS-CoV-2, and excess usage of Furin by the virus led to decreased activity of epithelial sodium channel; because of that, the retention of potassium and hyperkalemia may occur [28].
Serum potassium levels in our patients were significantly correlated with serum creatinine, BUN, PTT, INR, AST, and LDH that are expected to be elevated in patients with poor outcomes as those on mechanical ventilation, intractable persistent hypoxemia, or who develop multi-organ failure.
A previous study revealed that COVID-19 patients with a potassium level of 5.0 mmol/L exhibited a substantially higher 30-day death rate than those with potassium level of 4.0 to 4.5 mmol/L. In individuals with COVID-19, plasma potassium levels should be checked regularly and kept within safe limits [29].
Unknown pathophysiological mechanisms connect hyperkalemia to higher mortality in COVID-19 patients. However, the current findings could be explained in part by several ways. First, the electrical characteristics of the myocardium’s resting membrane potential are altered by potassium levels, which trigger ventricular arrhythmia to develop [30]. Additionally, hyperkalemia causes complete heart block and sinus arrest by lowering ventricular excitability. An increased risk of severe bradyarrhythmia is linked to too high serum potassium levels. [22]. Second, critically ill patients are more likely to have abnormal potassium levels because they often use medications that impact K + regulation and are frequently affected by organ dysfunction [31]. Third, an abnormally high potassium level in the blood might be an indication of an acid–base imbalance with severe acute respiratory distress syndrome [32].
The calcium is central to membrane fusion and the entrance of SRS COV virus [33, 34]. This study revealed that serum calcium level was mildly decreased in COVID-19 patients. Moreover, it is significantly lower in those who develop a worse prognosis, This is in concordance with a previous study, which revealed that serum calcium is a marker for COVID-19 severity and low serum calcium is an indicator of severe COVID-19 disease [35]; our study revealed that low serum calcium was significantly associated with high serum ferritin, CRP, creatinine, bilirubin, INR, and AST.
Despite the established role of magnesium in immune function and a proven connection between serum magnesium level and the release and regulation of inflammatory markers like interleukin 6 [36], our results showed that serum magnesium was normal at the time of patient admission and that there was no significant difference in serum magnesium detected between patients who develop a worse prognosis and those with good prognosis. Also, apart from LDH, no significant correlation between serum magnesium and the laboratory data of the patients was detected.
Regarding serum phosphorus level in our patients, it was normal at the time of admission but the patient who developed acute kidney injury or multi-organ failure developed hyperphosphatemia.
Our study has several limitations. It was carried out as a single-center study. Also, it was retrospective with some potential biases; the different medications taken by the patient before the critical care admission may affect the level of electrolytes, as some of our patients started treatment and oxygen at home and presented late to the hospital.
Conclusion
This report states that although the mean serum electrolytes of patients admitted to the ICU with COVID-19 infection were within normal limits, yet unfavorable admission course and mortality were significantly associated with higher serum sodium, potassium, phosphorus, and lower calcium levels. We recommend the check and proper correction of serum electrolytes at the time of admission of COVID-19 patients and serially during the hospital admission course.
Availability of data and materials
Data can be obtained from corresponding authors upon reasonable request.
Abbreviations
- COVID-19:
-
Coronavirus disease 2019
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- IL-6:
-
Interleukin 6
- ACE2:
-
Angiotensin I converting enzyme 2
- RAS:
-
Renin–angiotensin system
- ARDS:
-
Acute respiratory distress syndrome
- ICUs:
-
Intensive care units
- DM:
-
Diabetes mellitus
- AKI:
-
Acute kidney injury
- CBC:
-
Complete blood count
- KFT:
-
Kidney function test
- LFT:
-
Liver function test
- PT:
-
Prothrombin time
- PTT:
-
Partial thromboplastin time
- INR:
-
International normalized ratio
- CRP:
-
C-reactive protein
- ESR:
-
Erythrocyte sedimentation rate
- LDH:
-
Lactate dehydrogenase
- CT chest:
-
Computed tomography of the chest
References
Fehr AR, Perlman S (2015) Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol 1282:1–23. Available from: https://pubmed.ncbi.nlm.nih.gov/25720466/. [cited 2022 Feb 27]
Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al (2020) A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382(8):727–33. Available from: https://pubmed.ncbi.nlm.nih.gov/31978945/. [cited 2022 Feb 27]
Pourfridoni M, Abbasnia SM, Shafaei F, Razaviyan J, Heidari-Soureshjani R (2021) Fluid and electrolyte disturbances in COVID-19 and their complications. Biomed Res Int 2021:6667047. https://doi.org/10.1155/2021/6667047
Forero-Peña DA, Carrión-Nessi FS, Mendoza-Millán DL, Omaña-Ávila ÓD, Mejía-Bernard MD, Camejo-Ávila NA, et al (2022) First wave of COVID-19 in Venezuela: epidemiological, clinical, and paraclinical characteristics of first cases. J Med Virol 94(3):1175–85. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/jmv.27449. [cited 2022 Feb 27]
Tan L, Wang Q, Zhang D, Ding J, Huang Q, Tang YQ, et al (2020) Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther 5(1):1–3. Available from: https://www.nature.com/articles/s41392-020-0148-4. [cited 2022 Feb 27]
Henry BM, De Oliveira MHS, Benoit S, Plebani M, Lippi G (2020) Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med 58(7):1021–8. Available from: https://pubmed.ncbi.nlm.nih.gov/32286245/. [cited 2022 Feb 27]
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395(10223):497–506. Available from: http://www.thelancet.com/article/S0140673620301835/fulltext. [cited 2022 Feb 27]
Lippi G, South AM, Henry BM (2020) Electrolyte imbalances in patients with severe coronavirus disease 2019 (COVID-19). Ann Clin Biochem 57(3):262–5. Available from: https://pubmed.ncbi.nlm.nih.gov/32266828/. [cited 2022 Feb 27]
Lu R, Zhao X, Li J, Niu P, Yang B, Wu H et al (2020) Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 395(10224):565–574
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al (2020) Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA 323(11):1061–9. Available from: https://jamanetwork.com/journals/jama/fullarticle/2761044. [cited 2022 Feb 27]
Thongprayoon C, Cheungpasitporn W, Chewcharat A, Mao MA, Vallabhajosyula S, Bathini T, et al (2020) Risk of respiratory failure among hospitalized patients with various admission serum potassium levels. Hosp Pract (1995) 48(2):75–9. Available from: https://pubmed.ncbi.nlm.nih.gov/32063075/. [cited 2022 Jun 12]
Bielecka-Dabrowa A, Mikhailidis DP, Jones L, Rysz J, Aronow WS, Banach M (2012) The meaning of hypokalemia in heart failure. Int J Cardiol 158(1):12–7. Available from: https://pubmed.ncbi.nlm.nih.gov/21775000/. [cited 2022 Feb 27]
Hodax JK, Bialo SR, Yalcindag A (2018) SIADH in systemic JIA resolving after treatment with an IL-6 inhibitor. Pediatrics 141(1). Available from: https://pubmed.ncbi.nlm.nih.gov/29242269/. [cited 2022 Feb 27]
Berni A, Malandrino D, Parenti G, Maggi M, Poggesi L, Peri A (2020) Hyponatremia, IL-6, and SARS-CoV-2 (COVID-19) infection: may all fit together? J Endocrinol Invest 43(8):1137–9. Available from: https://pubmed.ncbi.nlm.nih.gov/32451971/. [cited 2022 Feb 27]
Booth CM, Matukas LM, Tomlinson GA, Rachlis AR, Rose DB, Dwosh HA, et al (2003) Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA 289(21):2801–9. Available from: https://pubmed.ncbi.nlm.nih.gov/12734147/. [cited 2022 Feb 27]
Masoud HH, Elassal G, Zaky S, Kamal E.K (2020) Management Protocol for COVID-19 Patients Version 1.4/30th May 2020 Ministry of health and population (MOHP), Egypt. In Coronavirus Disease 2019 (COVID-19); SARS COV2 Management Guide Line; Ministryof Health and Population: Cairo. Available online: http://www.mohp.gov.eg/JobsDetails.aspx?job_id=3061. (Accessed on 27 May 2021)
General Authority for Healtcare Accreditation and Regulation (GAHAR), Egypt (2021) GAHAR handbook for clinical laboratory standards. Version 2021. Published online and accessed at: https://gahar.gov.eg/upload/gahar-handbook-for-clinical-laboratories-standards-1.pdf
Habib MB, Sardar S, Sajid J (2020) Acute symptomatic hyponatremia in setting of SIADH as an isolated presentation of COVID-19. IDCases 21:e00859. https://doi.org/10.1016/j.idcr.2020.e00859
Sjöström A, Rysz S, Sjöström H, Höybye C (2021) Electrolyte and acid-base imbalance in severe COVID-19. Endocr Connect 10(7):805–14. Available from: https://pubmed.ncbi.nlm.nih.gov/34156969/. [cited 2022 Feb 27]
Zimmer MA, Zink AK, Weißer CW, Vogt U, Michelsen A, Priebe HJ, et al (2020) Hypernatremia-a manifestation of COVID-19: a case series. A&A Pract 14(9):e01295. Available from: https://pubmed.ncbi.nlm.nih.gov/32909725/. [cited 2022 Feb 27]
Tzoulis P, Waung JA, Bagkeris E, Hussein Z, Biddanda A, Cousins J, et al (2021) Dysnatremia is a predictor for morbidity and mortality in hospitalized patients with COVID-19. J Clin Endocrinol Metab 106(6):1637–48. Available from: https://pubmed.ncbi.nlm.nih.gov/33624101/. [cited 2022 Feb 27]
Tongyoo S, Viarasilpa T, Permpikul C (2018) Serum potassium levels and outcomes in critically ill patients in the medical intensive care unit. J Int Med Res 46(3):1254. Available from: /pmc/articles/PMC5972260/. [cited 2022 Feb 27].
Ferrer M, Travierso C, Cilloniz C, Gabarrus A, Ranzani OT, Polverino E, et al (2018) Severe community-acquired pneumonia: Characteristics and prognostic factors in ventilated and non-ventilated patients. PLoS One 13(1):e0191721. Available from: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0191721. [cited 2022 Feb 27].
Xi H, Yu RH, Wang N, Chen XZ, Zhang WC, Hong T (2019) Serum potassium levels and mortality of patients with acute myocardial infarction: a systematic review and meta-analysis of cohort studies. Eur J Prev Cardiol 26(2):145–56. Available from: https://pubmed.ncbi.nlm.nih.gov/31060369/. [cited 2022 Feb 27]
Engelhardt LJ, Balzer F, Müller MC, Grunow JJ, Spies CD, Christopher KB, et al (2019) Association between potassium concentrations, variability and supplementation, and in-hospital mortality in ICU patients: a retrospective analysis. Ann Intensive Care 9(1). Available from: https://pubmed.ncbi.nlm.nih.gov/31486927/. [cited 2022 Feb 27]
Bengalorkar GM, Sarala N, Venkatrathnamma PN, Kumar TN (2011) Effect of heparin and low-molecular weight heparin on serum potassium and sodium levels. J Pharmacol Pharmacother 2(4):266–9. Available from: https://pubmed.ncbi.nlm.nih.gov/22025855/. [cited 2022 Mar 6]
Torres OH, Hernandez N, Francia E, Barcelo M, Mateo J, Ruiz D (2010) Effect of prophylactic treatment with low-molecular-weight heparin bemiparin sodium on serum potassium levels: a prospective observational study. Drugs Aging 27(5):399–406. Available from: https://pubmed.ncbi.nlm.nih.gov/20450237/. [cited 2022 Mar 6]
Noori M, Nejadghaderi SA, Sullman MJM, Carson-Chahhoud K, Ardalan M, Kolahi AA, et al (2021) How SARS-CoV-2 might affect potassium balance via impairing epithelial sodium channels? Mol Biol Rep 48(9):6655–61. Available from: https://pubmed.ncbi.nlm.nih.gov/34392451/. [cited 2022 Mar 1]
Liu S, Zhang L, Weng H, Yang F, Jin H, Fan F et al (2021) Association between average plasma potassium levels and 30-day mortality during hospitalization in patients with covid-19 in wuhan, china. Int J Med Sci 18(3):736–743
Faxén J, Xu H, Evans M, Jernberg T, Szummer K, Carrero JJ (2019) Potassium levels and risk of in-hospital arrhythmias and mortality in patients admitted with suspected acute coronary syndrome. Int J Cardiol 274:52–8. Available from: https://pubmed.ncbi.nlm.nih.gov/30282599/. [cited 2022 Feb 27].
McMahon GM, Mendu ML, Gibbons FK, Christopher KB (2012) Association between hyperkalemia at critical care initiation and mortality. Intensive Care Med 38(11):1834–42. Available from: https://pubmed.ncbi.nlm.nih.gov/22806439/. [cited 2022 Feb 27]
Romano TG, Correia MDT, Mendes PV, Zampieri FG, Maciel AT, Park M (2016) Metabolic acid-base adaptation triggered by acute persistent hypercapnia in mechanically ventilated patients with acute respiratory distress syndrome. Rev Bras Ter intensiva 28(1):19–26. Available from: https://pubmed.ncbi.nlm.nih.gov/27096672/. [cited 2022 Feb 27]
Millet JK, Whittaker GR (2018) Physiological and molecular triggers for SARS-CoV membrane fusion and entry into host cells. Virology 517:3–8. Available from: https://pubmed.ncbi.nlm.nih.gov/29275820/. [cited 2022 Feb 27]
Straus MR, Tang T, Lai AL, Flegel A, Bidon M, Freed JH, et al (2020) Ca 2+ Ions Promote Fusion of Middle East Respiratory Syndrome Coronavirus with Host Cells and Increase Infectivity. J Virol 94(13). Available from: https://pubmed.ncbi.nlm.nih.gov/32295925/. [cited 2022 Feb 27]
Zhou X, Chen D, Wang L, Zhao Y, Wei L, Chen Z, et al (2020) Low serum calcium: a new, important indicator of COVID-19 patients from mild/moderate to severe/critical. Biosci Rep 40(12). Available from: https://pubmed.ncbi.nlm.nih.gov/33252122/. [cited 2022 Feb 27]
Sarvazad H, Cahngaripour SH, Eskandari Roozbahani N, Izadi B (2020) Evaluation of electrolyte status of sodium, potassium and magnesium, and fasting blood sugar at the initial admission of individuals with COVID-19 without underlying disease in Golestan Hospital, Kermanshah. New microbes new Infect 38. Available from: https://pubmed.ncbi.nlm.nih.gov/33294198/. [cited 2022 Feb 27]
Acknowledgements
We thank the Isolation Hospital ICU staff at Zagazig University Hospital for providing the necessary data to conduct this study.
Funding
No any specific financial interests, relationship, and affiliations relevant to the subjects of the manuscript.
Author information
Authors and Affiliations
Contributions
E.N., M.S., and T.H. contributed to the conception and design of the study. M.H., M.M., A.A., and D.A. organized the database. E.N. and A.T. performed the statistical analysis. E.N. and M.S. plotted the figures and tables in this work. E.N., M.S., and T.H. wrote the first draft of the manuscript. M.H., M.M., and D.A. wrote the sections of the manuscript. All authors contributed to the manuscript revision and read and approved the submitted version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Our study was approved by the Institutional Review Boards of Faculty of Medicine, Zagazig University, with the reference number ZU-IRB#: 6328–23-8–2020, and it was registered with ClinicalTrials.gov (NCT04539834) at (07/09/2020). Data collection is retrospective and anonymous. Therefore, the Institutional Review Boards of the Affiliated Hospital of Zagazig University waived the need for informed consent. All methods in this study were carried out in accordance with relevant guidelines and regulations (the Declarations of Helsinki).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mohamed, M.S., Negm, E.M., Zahran, M.H. et al. Electrolyte profile in COVID-19 patients: insights into outcomes. Egypt J Bronchol 17, 48 (2023). https://doi.org/10.1186/s43168-023-00225-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43168-023-00225-2