Abstract
Background
Extracorporeal membrane oxygenation (ECMO) can be used as salvage therapy for multitrauma patients with acute respiratory distress syndrome (ARDS) when conventional treatment fails to maintain oxygenation. However, controversy exists between ECMO application and the risk of bleeding due to systemic anticoagulation during the treatment. Non-heparin introduction seems to be a possible solution for this dilemma, owing to technical improvements in the device and management methods of ECMO.
Case presentation
A 58-year-old woman suffered from blunt thoracic, pelvic, and right lower limb fractures due to a falling accident, which resulted in acute respiratory distress syndrome (ARDS). Although the patient received intubation and mechanical ventilation (MV), respiratory failure was not alleviated. Venous-venous (V-V) ECMO was used as a salvage therapy. With the support of V-V ECMO, we safely cleared blood clots in the bronchus and avoided secondary lung injury caused by pressure trauma and oxygen poisoning of the MV. We introduced heparin-free ECMO management as a solution to reduce the risk of bleeding associated with pulmonary contusion and other organ injuries. To prevent thrombosis, we set the blood rate of ECMO to 4.0 L/min, which is much higher than the usual parameter. During ECMO, coagulation factors, such as prothrombin time, activated partial thromboplastin time, and D-dimer, were examined. ECMO was maintained for 5 days without any complications; MV was stopped on the 13th day, extubated on the 24th day, and discharged from ICU on the 28th day.
Conclusion
ECMO with non-heparin could be an optimal treatment for multitrauma patients with ARDS when traditional treatment cannot sustain oxygenation. High blood flow rate could prevent thrombosis through ongoing ECMO therapy without systemic anticoagulation. In addition, monitoring D-dimer value change (Δ D-dimer) may be better than D-dimer value in predicting clot formation in the membrane oxygenator.
Similar content being viewed by others
Background
Multitrauma is usually a life-threatening emergency and often caused by vehicular accidents or fall. Blunt thoracic trauma is reported to be associated with 50% of multitrauma cases, of which 75% lead to pulmonary contusion [1, 2]. Pulmonary edema and alveolar hematoma, which induced pulmonary contusion, are the two main reasons for acute respiratory distress syndrome (ARDS) [3]. Intubation and mechanical ventilation are traditional treatments for ARDS, but worsening lung compliance and high inspiratory pressure lead to further lung injury. When conventional mechanical ventilation therapy failed, extracorporeal membrane oxygenation (ECMO) may be the final rescue therapy, which can provide adequate oxygenation with low positive pressure and oxygenation supply of ventilator [4].
Although ECMO has successfully rescued ARDS patient of trauma in 1971 at the Cottage Hospital in Santa Barbara, the auxiliary role of ECMO in trauma patients is still controversial because of further bleeding in multiple trauma patients secondary to systemic anticoagulation in ECMO treatment. Recently, ECMO technology has made great progress, including heparin-coated circuit and polymethylpentene oxygenator, which may reduce the anticoagulation requirements in some clinical scenarios [5]. Therefore, heparin-free ECMO seems to be a feasible method to solve the problem of high risk of bleeding in multiple trauma patients who need ECMO support. Without system anticoagulation, high blood flow rate of ECMO is set to prevent clot formation in membrane oxygenator (MO) [6, 7]. Although the progress of ECMO device and the management strategy of high blood flow have reduced thrombosis, the clot formation in ECMO device cannot be completely avoided. As a predictor of thrombosis, D-dimer could predict thrombosis for bedside surveillance of MO and to avoid system-induced coagulation disorders [8].
At present, studies on how to set the blood flow velocity of ECMO to prevent thrombosis and forecast the D-dimer value in ECMO device in detail to predict thrombosis during treatment are few. Here, we reported our experience of non-heparinized ECMO management strategies in a multitrauma patient with respiratory failure due to hemothorax, pulmonary contusion, and airway obstruction by blood clots.
Case presentation
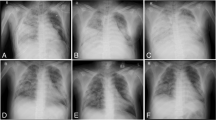
A 58-year-old woman suffered from blunt thoracic, pelvic fracture, and right lower limb fracture because of falling from approximately 6 m high by accident. She was transferred to the nearest hospital and admitted into the intensive care unit (ICU) immediately. Emergency CT examination showed bilateral pulmonary contusion, right pleural effusion, bilateral multiple rib fractures, right clavicle fractures, lumbar two–four vertebral spinous process fractures, right iliac bone fractures, and right femoral shaft fractures. The patient received single-lumen intubation and mechanical ventilation (MV) because of refractory tachypnea and hypoxemia. In addition, chest X-ray showed a right large hemothorax with atelectasis (Fig. 1). The patient presented hemorrhagic shock due to hemothorax and multiple fractures. The patient was treated with closed drainage of the right thoracic cavity, enhanced MV support, fixation and traction of the right lower limb, and fluid and blood transfusion resuscitation. After these treatments, blood pressure could be maintained with low-dose vasopressors, but no alleviation of hypoxemia was observed. Therefore, the patient was transferred to our ICU department on day 2.
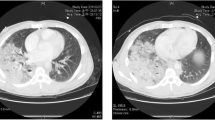
On arrival, the patient had severe dyspnea with paradoxical breathing, respiratory rate at 35 breaths per minute, tachycardia at 130 beats per minute, and arterial pressure of 122/68 mmHg with norepinephrine infusion at 0.1 μg/kg/min. Breathing sounds decreased, oxygenation saturation was 90% with ventilator set as fraction of inspiration oxygenation (FiO2) 1.0, pressure control (PC) 25 cmH2O, positive end-expiratory pressure (PEEP) 15 cmH2O, and tidal volume (VT) 300 ml; arterial blood gas analysis showed that pH was 7.32, PaO2 was 53 mmHg, and PCO2 was 72 mmHg. Meanwhile, the bedside bronchoscopy showed that the main bronchus was almost obstructed by foreign bodies, which may be blood clots caused by bronchial hemorrhage (Fig. 2). When the PaO2/FiO2 ratio (PFR) was 53 mmHg, removing the blood clots was dangerous, and threatened bleeding would recur after blood clots were removed. Moreover, mechanical ventilation and respiratory distress can aggravate lung injury and lead to persistent pulmonary hemorrhage. Therefore, we decided to establish veno-venous (V-V)-ECMO, and insert catheter via the right jugular vein (19-F cannula for inflow) located at the right atrium and the right femoral vein (21-F cannula for outflow) located at the inferior vena cava. Blood was drawn out from the inferior vena cava to the ECMO device (Maquet, ROTAFLOW Console), and it was infused into the right atrium after oxygenation. We did not use any anticoagulation drugs because of bronchial bleeding, right hemothorax, and other unstable organ injuries. The pump was set at 3800–4000 rpm to maintain a blood flow rate of approximately 4.0 L/min, which may prevent thrombus formation, and the gas flow rate at 3–5 L/min and FiO2 1.0. After ECMO was established, mechanical ventilation parameter was declined to prevent barotrauma with remediation of respiratory distress. Arterial blood gas analysis showed that pH was 7.45, PaO2 was 163 mmHg, and PCO2 was 30 mmHg with ventilator set as PC 14 cmH2O, PEEP 10 cmH2O, VT 200 ml, and FiO2 0.4. The patient was stable with respiratory rate of 12 breaths per minute, heart rate of 80 beats per minute, and arterial blood pressure of 110/60 mmHg without any vasopressor. Further CT scan showed that the bilateral lung consolidation and trachea were almost obstructed completely (Fig. 3). With ECMO support, we performed bronchoscopy(electronic bronchoscope type: Olympus BF-1TQ290) with carbon dioxide cryotherapy (equipment: Erbe Elektromedizin GmbH) to clear the foreign bodies in the main segments of bronchopulmonary, and the pathology results showed that the foreign bodies were blood clots (Fig. 4).
When the clots were removed, the ventilator parameters were the same as the previously set condition, and VT was augmented from 200 ml to nearly 400 ml. Chest CT scan was obtained prior to the withdrawal of ECMO, showing that the clots in the trachea disappeared and pulmonary atelectasis improved (Fig. 5). We weaned ECMO support by decreasing oxygen supply on day 3 and removing ECMO when the oxygen supply stopped for 24 h on day 6 with ventilator set to PC 13 cmH2O, PEEP 8 cmH2O, and PFR > 300 mmHg because of non-heparinization. No ECMO-related infection and bleeding were observed during the course. Prothrombin time (PT), activated partial thromboplastin time (APTT), and D-dimer were monitored four times each day during ECMO ongoing to evaluate ECMO thrombogenicity. We removed the right thoracic drainage tube on day 5 and the left thoracic drainage tube on day 6. The patient underwent tracheostomy on day 10, MV stopped on day 13, and bronchial bleeding totally stopped on day 15. The right femoral shaft and pelvic fracture underwent repositioning and osteosynthesis under general anesthesia on day 18. The patient was extubated on day 24, transferred to the traumatology ward on day 28, and discharged on day 35. Besides, the tracheostoma of the patient healed by itself in 3 days which did not need to be stitched.
Discussion
This report described a case about heparin-free ECMO established to rescue the patient with acute respiratory failure caused by lung injury and airway obstruction. Removing blood clots by bronchoscopy under severe respiratory failure was dangerous. Otherwise, bleeding would recur when blood clot was removed, inducing asphyxia to death. V-V ECMO modality was selected to alleviate respiratory failure, tissue hypoxia, and CO2 removal given that the patient had no potential heart disease and the ultrasonic cardiogram was normal. With the support of ECMO and mechanical ventilation, the blood clots were removed by bronchoscopy with cryotherapy in safe, and the patient survived from acute respiratory failure. We introduce this case in detail, to guide the treatment of similar cases in the future, especially the patients who need V-V ECMO support with active bleeding.
Multitrauma with blunt thoracic injuries usually experiences ARDS [9]. Notwithstanding the therapeutic advances that improved outcomes in ARDS [10], lung injury remains significant with a high risk of mortality, especially concurrent severe chest trauma and hemorrhage. In this situation, ECMO could be the salvage therapy to trauma patient with severe respiratory failure [4]. It may provide adequate tissue perfusion, oxygenation, and ventilation support reduction to prevent further ventilator-associated lung injury and provide time for lung recovery. However, ECMO remains controversial in patients with multiple injuries associated with lung failure and high risk of bleeding.
The improvement of ECMO technique in circuit (coated with heparin) and pump material polymethylpentene decreased thrombogenicity and reduced hemorrhagic complication by mitigating anticoagulation requirements [11, 12]. A 10-year study reported that 94% of ECMO cases were anticoagulated with heparin successfully [13]. However, some patients had to stop heparin injection due to severe bleeding, even though the anticoagulation effect was good [7].
With regard to the patient who had hemothorax, pelvic fracture, and femoral shaft fracture, we still selected the heparin-free management to reduce the risk of bleeding. However, no systemic heparin administration had a potentially higher risk for thrombosis of the MO. During this period, monitoring coagulation function factors, such as APTT, PT, and D-dimer is important. Especially, D-dimer is a sensitive marker for thrombus formation; it was used effectively to evaluate clot formation in MO. Christian reported that [8] D-dimer levels were positively correlated with clot volume of MO, which was calculated using multidetector computed tomography. In this case, PT and APTT were normal and stable, and D-dimer was at a high level prior to ECMO application and gradually increased in the first 2 days after ECMO (Fig. 6). We also observed three small clots that formed in the MO. They indicated that clot was formed in the MO because of non-system anticoagulation. In this case, we increased the blood flow from 3.0 L/min to no less than 4.0 L/min, which is higher than in other studies [6, 14], and no thrombosis was founded in MO. Compared with other studies, higher blood flow rate is required to prevent clot formation because of an ideal flow rate for each MO [6]. D-dimer value deescalated to the baseline (value of D-dimer prior to ECMO application), but it was still over the cut-off value for thrombus formation compared with other studies [8]. However, thrombus formation was not observed during ECMO period because the D-dimer value was high prior to ECMO implantation and did not return to normal level after ECMO therapy until we repaired the broken right leg and pelvis. We inferred that ECMO and multitrauma affected the D-dimer value. In this situation, we speculate that the D-dimer value changed (Δ D-dimer) to a better parameter to evaluate ECMO thrombogenicity, and a large sample is required to further characterize this relationship.
Conclusion
Heparin-free ECMO could be an available option for multitrauma patient with active bleeding when conventional ventilation support does not work. High blood flow rate could prevent thrombosis for ECMO with non-heparin anticoagulation, and D-dimer could be a more useful marker to evaluate thrombus formation in some conditions.
Availability of data and materials
No more data available.
Patient gave consent for publication and consent for publication was written.
Abbreviations
- ARDS:
-
Acute respiratory distress syndrome
- ECMO:
-
Extracorporeal membrane oxygenation
- ICU:
-
Intensive care unit
- CT:
-
Computerized tomography
- MV:
-
Mechanical ventilation
- PT:
-
Prothrombin time
- APTT:
-
Activated partial thromboplastin time
- FiO2:
-
Fraction of inspiration oxygenation
- PC:
-
Pressure control
- PEEP:
-
Positive end-expiratory pressure
- VT:
-
Tidal volume
- PFR:
-
PaO2/FiO2 ratio
- MO:
-
Membrane oxygenator
References
Ried M, Bein T, Philipp A, Muller T, Graf B, Schmid C, Zonies D, Diez C, Hofmann HS (2013) Extracorporeal lung support in trauma patients with severe chest injury and acute lung failure: a 10-year institutional experience. Crit Care 17(3):R110
Alisha C, Gajanan G, Jyothi H (2015) Risk factors affecting the prognosis in patients with pulmonary contusion following chest trauma. J Clin Diagn Res 9(8):OC17-9
Szilárd R, Tamás FM (2019) Pulmonary contusion. J Thorac Dis 11(Suppl 2):S141–S151
Tonna JE, Abrams D, Brodie D, Greenwood JC, Rubio Mateo-Sidron JA, Usman A, Fan E (2021) Management of adult patients supported with venovenous extracorporeal membrane oxygenation (VV ECMO): guideline from the Extracorporeal Life Support Organization (ELSO). ASAIO J 67(6):601–610
Mesher AL, McMullan DM (2014) Extracorporeal life support for the neonatal cardiac patient: outcomes and new directions. Semin Perinatol 38(2):97–103
Ki KK, Passmore MR, Chan CHH, Malfertheiner MV, Fanning JP, Bouquet M, Millar JE, Fraser JF, Suen JY (2019) Low flow rate alters haemostatic parameters in an ex-vivo extracorporeal membrane oxygenation circuit. Intensive Care Med Exp 7(1):51
Ogawa F, Sakai T, Takahashi K, Kato M, Yamaguchi K, Okazaki S, Abe T, Iwashita M, Takeuchi I (2019) A case report: Veno-venous extracorporeal membrane oxygenation for severe blunt thoracic trauma. J Cardiothorac Surg 14(1):88
Dornia C, Philipp A, Bauer S, Stroszczynski C, Schreyer AG, Muller T, Koehl GE (2015) D-dimers are a predictor of clot volume inside membrane oxygenators during extracorporeal membrane oxygenation. Artif Organs 39(9):782–787
Blondonnet R, Begard M, Jabaudon M, Godet T, Rieu B, Audard J, Lagarde K, Futier E, Pereira B, Bouzat P, Constantin JM (2021) Blunt chest trauma and regional anesthesia for analgesia of multitrauma patients in French intensive care units: a national survey. Anesth Analg 133(3):723–730
Peck TJ, Hibbert KA (2019) Recent advances in the understanding and management of ARDS. F1000Res 8:F1000 (Faculty Rev-1959)
Wen PH, Chan WH, Chen YC, Chen YL, Chan CP, Lin PY (2015) Non-heparinized ECMO serves a rescue method in a multitrauma patient combining pulmonary contusion and nonoperative internal bleeding: a case report and literature review. World J Emerg Surg 10:15
Arlt M, Philipp A, Voelkel S, Leopold R, Thomas M, Michael H, Bernhard MG, Christof S (2010) Extracorporeal membrane oxygenation in severe trauma patients with bleeding shock. Resuscitation 81(7):804–809
Ahmad SB, Menaker J, Kufera J, O’Connor J, Scalea TM, Stein DM (2017) Extracorporeal membrane oxygenation after traumatic injury. J Trauma Acute Care Surg 82(3):587–591
Lorini FL, Grazioli L, Manfredi R, Rausa E, Ghitti D, Poli G, Peck M, Cattaneo S (2020) A prolonged and successful heparin-free extracorporeal membrane oxygenation run in isolated thoracic trauma: A case report. Int J Artif Organs 43(4):288–291
Acknowledgements
The authors thank ShineWrite for the language support.
Funding
No funding support.
Author information
Authors and Affiliations
Contributions
QM was a major contributor in writing the manuscript. CJ and LC are two respiratory therapists; they were in charge of management ventilator and ECMO device. ZM, BC, and HZ established V-V ECMO and inserted catheter via the right jugular vein and the right femoral vein. JL collected and analyzed clinical data. SS and FQ made a decision on therapy strategies. All authors read and approved the final manuscript.
Authors’ information
None.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable (only standard care were performed).
Consent for publication
Patient gave consent for publication and consent for publication was written.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Meng, Q., Ji, C., Ma, Z. et al. A case report: extracorporeal membrane oxygenation for multitrauma patient with pneumorrhagia. Egypt J Bronchol 17, 9 (2023). https://doi.org/10.1186/s43168-023-00182-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43168-023-00182-w