Abstract
Background
Severe asthma exacerbation can be a frightening experience to the patient and physician. Despite continuous efforts to frame management guidelines and advances in treatment, severe exacerbations still occur. In order to prevent and judicious management of asthma exacerbations, we should predict them first. This study aims to evaluate distinct clinical trajectories and management outcome of patients with severe asthma exacerbations and also evaluate predictors for poor outcome.
Methods
Patients suffering from acute asthma exacerbation and presented to emergency room (forty patients) were grouped into 2 groups (groups A and B) according to severity of exacerbation. Assessment included full clinical history, laboratory investigations (including eosinophil cell count and serum IgE level), Beck’s anxiety and depression inventory scales, assessment of asthma medication adherence and control level, and peak expiratory flow measurement (at presentation, 1 and 6 h after).
Results
Fifty-five percent of patients suffered from severe and life-threatening asthma exacerbations, 63.6% of them were females. The most important predictors for severe exacerbations were SO2 < 90% at baseline (OR = 4.56; 95% CI = 3.45–7.56; P < 0.001), PEFR after 1 h (OR= 3.34; 95%CI = 1.90–4.90; P < 0.001), and uncontrolled asthma (OR= 3.33; 95%CI = 2.50–5.05; P < 0.001). Predictors for hospitalization were old age (OR = 1.11; 95%CI = 1.09–2.11; P < 0.001), uncontrolled asthma (OR = 2.34; 95%CI = 2.01–4.40; P < 0.001), PEFR after 1 h (OR= 4.44; 95%CI= 3.24–7.68; P < 0.001), and SO2 <90% at baseline (OR= 5.67; 95%CI= 3.98–8.50; P < 0.001).
Conclusions
Severe asthma exacerbations can be predicted by old age, previous history of mechanical ventilation, obstructive sleep apnea, overuse of SABA, uncontrolled asthma, moderate to severe depression, eosinophilia, SO2 <90%, and low peak expiratory flow rates.
Similar content being viewed by others
Background
Asthma is a common and potentially serious chronic disease that imposes a substantial burden on patients, their families, and the community. It causes respiratory symptoms, limitation of activity, and flare-ups (attacks) that sometimes require urgent health care and may be fatal [1].
Severe exacerbation can occur in patients with mild or well-controlled asthma. Exacerbations may occur in patients with a pre-existing diagnosis of asthma or, occasionally, as the first presentation of asthma in response to exposure to an external agent risk factor (e.g., viral upper respiratory tract infection, pollen, or pollution) and/or poor adherence with controller medication; however, a subset of patients presents more acutely and without exposure to known risk factors [2].
Acute severe asthma is considered a major economic & health burden. It represents about 3% of hospital admissions [3]. Some other entities similar not identical to that of acute severe asthma also require precise definitions: so Kenyon et al. [4] proposed the term critical asthma syndromes (CAS) to identify any child or adult who is at risk of fatal asthma.
Around 300 million people have asthma worldwide (likely by 2025, a further 100 million may be affected) and account for 1 in every 250 deaths [5]. Acute severe asthma attack is increasingly associated with different specific phenotypes and it represents a major unmet therapeutic need [6].
Asthma critically depends on a series of cell adhesion molecule-mediated interactions between vascular endothelium and leukocytes especially associated with T-helper cell type 2 (Th2) immune responses, which are typical of other atopic conditions. Elevated levels of Th2 cells in the airways release specific cytokines, including interleukin IL-4, IL-5, IL-9, and IL-13 that activate B lymphocytes to produce allergen-specific IgE which binds to the high affinity mast cell receptors, leading to their activation and the release of inflammatory mediators as histamine [7, 8].
This study aims to evaluate the management outcome of patients with severe asthma exacerbations and also evaluate predictors for both hospitalization and poor outcome.
Patients and methods
Study participants and ethical approval
This prospective observational study was performed on 40 asthmatic patients in the age group of 18–70 years suffering from asthma exacerbation who were identified using the diagnostic criteria defined by Global Initiative for Asthma [9] belonging to either gender and referred to emergency department of Assiut university hospital during the period from October 2019 to October 2020. Severe asthma exacerbation was considered for a patient who talks in words, is agitated, uses accessory respiratory muscles, and has a respiratory rate > 30/min, heart rate > 120/min, O2 saturation on air < 90%, and PEF ≤ 50% of their best or predicted value [9].
Patients were categorized according to exacerbation severity into group A including mild and moderate asthma exacerbation and group B including severe and life-threatening asthma exacerbation. All participants or their legal guardians gave informed written consent. Exclusion criteria were pregnancy, presence of other chronic pulmonary diseases, evidence of heart failure, pneumonia, and inability to obtain written informed consent. Study protocol was approved by the ethical committee of Faculty of Medicine, Assiut University, and it was carried out in accordance with the Declaration of Helsinki (IRB: 17100097).
Demographic, clinical, and laboratory data
Included patients underwent careful history taking and full clinical examination. Chest radiography to exclude comorbid conditions, complications, and mimics of asthma (pneumonia, pneumothorax, and heart failure). Medical care for asthma, number of prior hospitalizations for asthma, past mechanical ventilation for asthma, any psychic disturbance, hospitalization for asthma in the past 12 months, and past ED visits for asthma in the past 12 months were assessed.
Comorbidities as diabetes, hypertension, gastro-esophageal reflux disease (GERD), allergic rhino-sinusitis (AR), obstructive sleep apnea (OSA), and confirmed food allergy were assessed. Assessment of asthma medication adherence was done using Morisky Medication Adherence Scales (MMAS-4) in which a questionnaire from relatives of the patient was taken [10], as well as assessment of asthma control level [11]. Arterial blood gases (ABG): ABG was obtained on room air using heparinized blood sample from radial artery and analyzed using blood gases analyzer (Rapid lab 850; CHIRON /Diagnostics, Critical care systems). Complete blood count (CBC): Using CELL-DYN Ruby, 2ml of venous blood was placed in standard tubes containing K3 EDTA anticoagulant. Total RBCs, HB, total WBCs, eosinophils, platelet count, and random distribution width (RDW) were assessed. Serum non-specific IgE level was measured for all participants.
Assessment of anxiety and depression
Beck’s Anxiety Inventory (BAI)
This 21-item self-report questionnaire was originally developed to assess clinical anxiety differentiated from normal anxiety. The Beck Anxiety Inventory Scores Interpretation: A grand sum between 0 and 21 indicates low anxiety, grand sum between 22 and 35 indicates moderate anxiety, and grand sum that exceeds 36 is high [12].
Beck Depression Inventory 2 (BDI-II)
A 4-point scale indicates degree of severity; items are rated from 0 (not at all) to 3 (extreme form of each symptom). The Beck Depression Inventory Scores Interpretation: minimal range (0–13), mild depression (14–19), moderate depression (20–28), and severe depression (29–63) [13].
Peak expiratory flow measurement
Serial measurement of lung function by using Microlife Digital Peak Flow Meter (PEF) performed at presentation and again 1 to 6 h after initial treatment for categorizing the severity of the exacerbation and predicting the need for hospitalization.
Statistical analysis
Data was collected and analyzed using SPSS (statistical package for social sciences) program (version 20, IMB and Armonk, New York). Continuous data was expressed in the form of mean (± SD) and compared by t-test while nominal data was expressed in the form of frequency (percentage) and compared by chi-square test. Predictors of asthma exacerbation and hospitalization among enrolled patients were determined by regression analysis. Accuracy of PEF in prediction of acute severe asthma was assessed by receiver operator characteristics curve (ROC curve). Odds ratios were provided with 95% confidence intervals (CI), and hence, P-value was considered significant if <0.05.
Results
Forty-five percent of patients (group A) presented with mild and moderate exacerbations while 55% (group B) had severe and life-threatening exacerbation. Demographic data and patient characteristics are shown in Table 1.
Patients of group B had significantly higher age and body mass index (BMI) in comparison to those of group A [50.32 ± 10.92 Vs. 36.78 ± 8.86; P < 0.001 and 28.64 ± 3.86 Vs. 26.33 ± 2.76 (kg/m2); P = 0.04].
Patients with severe and life-threatening exacerbation (group B) had higher frequency of previous emergency room visits (100% vs. 66.7%; P < 0.001), hospitalization (86.4% vs. 33.3%; P< 0.001), and mechanical ventilation (50% vs. 11.1%; P = 0.01).
Moderate and high anxiety levels were prevalent in patients of group B. Mild, moderate, and severe depression was significantly higher in group B patients 40.9%, 50%, and 9.1%, respectively (Table 2).
Regarding laboratory data, severe group showed significantly lower SO2 (P< 0.001), higher eosinophil count (P< 0.001), and higher serum IgE level (P < 0.001) (Table 3). 61.1% patients of non-severe group vs. 18.2% of severe group were discharged. Frequency of hospitalization was 33.3% in the case of non-severe group vs. 72.7% in the case of severe group (Table 4).
Regarding the assessment of PEFR accuracy in the prediction of exacerbation severity among enrolled patients, PEFR after 1 h at cut-off point < 110 had 100% sensitivity and 62% specificity for the prediction of severe acute asthma with an overall accuracy of 82.9% and area under the curve was 0.81 (Tables 5 and 6); the predictors for severe exacerbation were old age, previous history of mechanical ventilation, obstructive sleep apnea, overuse of SABA, uncontrolled asthma, moderate to severe depression, eosinophilia, SO <90%, and low peak expiratory flow rates (Table 7).
The most important predictors were SO2 <90% at baseline (OR= 4.56; 95%CI= 3.45–7.56; P < 0.001), PEFR after 1 h (OR= 3.34; 95%CI= 1.90–4.90; P< 0.001), and uncontrolled asthma (OR= 3.33; 95%CI= 2.50–5.05; P< 0.001).
Based on the current study, the predictors of hospitalization were old age (OR= 1.11; 95%CI= 1.09–2.11; P < 0.001), uncontrolled asthma (OR= 2.34; 95%CI= 2.01–4.40; P < 0.001), PEFR after 1 h (OR= 4.44; 95%CI= 3.24–7.68; P < 0.001), and SO2 <90% at baseline (OR= 5.67; 95%CI= 3.98–8.50; P < 0.001) (Table 8).
Discussion
In this study, the mean age of patients of the severe group was 50.32 ± 10.92 significantly higher compared to 36.78 ± 8.86 for the non-severe group. Patient’s age >40 years was detected to be a significant predictor for exacerbation severity. That risk is mostly attributed to more comorbidities together with gradual decrease of lung function which will adversely affect asthma prognosis.
Most of the patients enrolled were females and most of group B were females. BMI was significantly higher in group B with mean values of 28.64 ± 3.86 vs. 26.33 ± 2.76 for group A. Percentage of current, passive, and ex-smokers were higher in group B.
Loymans et al. in their quest to develop a multivariate prediction model revealed that patients with exacerbations showed higher mean age (42.1 Vs 39) and higher percentage of females (77.5 vs 67) versus patients without exacerbations during a 12-month follow-up period [14].
On the contrary, Kang et al. [15] stated that frequent hospitalization history and poor drug adherence are the main factors responsible for severe exacerbations while patients with mild asthma require greater attention to their age and comorbidities.
Medical history showed that previous ED visit, hospitalization, and previous mechanical ventilation were significantly higher among patients of group B. Yii et al. [16] followed 177 patients with problematic asthma for 5 years; risk factors included two or more exacerbations in the last year, elevated body mass index, obstructive sleep apnea, depression, and GERD. 9.5% had frequent severe exacerbations [16].
Regarding previous hospital admission, our study confirmed it as a risk factor for future severe asthma exacerbation which is proved also by Alvarez et al. (2005) showing that a history of previous hospitalizations due to asthma exacerbations is independent predictor of near-fatal and fatal asthma [17]. Also Gonzalez-Barcala et al. [18] showed that the combination of previous hospital admissions and new episodes of hospitalization is consistently observed.
According to Silverman et al. [19], approximately 36% of emergency department patients with asthma aggravation were current smokers. Exposure to environmental tobacco smoke (ETS) has been cited as a risk factor for asthma exacerbations and emergency room visits and has been associated with worse asthma severity according to Merianos et al. [20].
Retrospective observational study by To et al. [21] showed that obesity was demonstrated to be independently and closely associated with severe acute exacerbations of asthma.
Obesity and asthma overlap with each other continuously and many phenotypes were described. Holguin et al. [22] stated that earlier onset asthma associated with obesity mostly suffer severe course (tended to have higher markers of Th2 inflammation). There is also a group with later-onset disease, mostly females, with a little airway inflammation, but significant inflammation in adipose tissue and increased airway oxidative stress [23]. The most notable changes caused by obesity in lung physiology are shown in a reduction of FRC and ERV.
In contrast to previous reports, a study by Kimura et al. [24] showed that the presence of several asthma comorbidities, such as obesity and GERD, was not associated with exacerbation status. These discrepancies may be explained by the ethnic differences between the study’s samples (i.e., lower prevalence of obesity and/or GERD).
OSA was one of the predictors of acute severe asthma exacerbation according to our study with an odds ratio of 1.11 and a 95% confidence interval of 1.01–2.35. Also Belachew et al. [25] concluded that obstructive sleep apnea was considerably linked with repeated exacerbations of asthma and the most prevalent among significantly associated predictors.
SABA overuse is considered a significant risk factor for severe asthma exacerbation that could be explained by the fact that the types of asthma that are uncontrolled or severe require more SABA use to relieve the frequent spasms and predicts that future exacerbations will be of more severity. UK registry data suggested SABA overuse or overreliance may be linked to asthma-related deaths [26].
Data from 1778 asthma patients attending primary care and specialist clinic were analyzed revealing that 66.2 % were poorly controlled, SABA overuse found in 26.2% who were prescribed ≥10 canisters per year. Findings from this African cohort of the SABINA III study indicate that SABA over-prescription and SABA over-the-counter purchase are common and associated with poor asthma-related outcomes. This highlights the need for healthcare providers/policymakers to align clinical practices with the latest treatment recommendations [27].
In terms of asthma control level, our study validated that patients of severe exacerbation group were significantly uncontrolled which made them more prone to asthma exacerbation severity and thus hospitalization. Also, Neffen et al. [28] found that 31% of patients had severe asthma, and of these, 64.1% were uncontrolled. Asthma control level remains unsatisfactory among most asthma patients; variables associated with poor control included non-adherent to medication (OR = 0.16, 95%CI (0.059, 0.48)), low level of patient enablement (OR = 0.19, (95%CI (0.08, 0.49)), and poor relationship with healthcare provider (OR = 0.024, 95%CI (0.02, 0.23)). Belachew et al. [29] highlighted multifaceted interventions, including comprehensive asthma education along with an integrated treatment plan to improve asthma control and quality of life.
High asthma adherence was found to significantly affects the exacerbation severity in this study which is explained by the fact that there may be deficient asthma management for group B patients and that illiterate patients are more in group B who are non-comprehensive of the proper use of ICS inhaler techniques mostly and are not aware of the importance of disease follow-up visits to the doctors. A cohort study by Sideleva et al. [23] concluded that most of the subjects with severe exacerbation adhered well to medicines; one of the explanations for the discrepancy is that patients with poor medication adherence were carefully excluded.
Regarding psychological impact on asthma exacerbation severity, we concluded that clinically concerning levels of anxiety and depression significantly and inversely affect patients of group B in this study and considered as a predictor for severe asthma attacks. Our study showed that female patients suffered clinically concerning scores of anxiety and depression more than males.
Most studies regarding asthma and anxiety and depression are cross-sectional; however, longitudinal studies have confirmed that the correlation between psychological abnormalities and asthma is stable over time. This could be explained by the following hypotheses: asthma itself increases the risk of developing anxiety and depression, mood and anxiety disorders lead to a higher risk of developing asthma, and asthma, anxiety, and depression are linked by a common underlying pathway [30].
Also, a cross-sectional study by Ritz et al. [31] confirmed that psychological triggers were consistently associated with exacerbations and emergency treatments over and above other triggers and affective disorders.
We investigated potential biomarkers related to Th2-driven inflammatory pathways, such as blood eosinophil count as it is a long-standing characteristic of asthma. In our study, higher eosinophilia (a blood eosinophil count > 300 cells/μL) was among group B versus group A [13 (59%) vs 1 (5.6%); p <0.001]. Zeiger et al. [32] likewise concluded that a blood eosinophil count > 400 cells/μL was an independent risk factor for asthma exacerbations and asthma-related emergency department visits or hospitalizations. Also, Jackson et al. [33] concluded the same results regarding eosinophil count, while serum IgE concentrations had no influence on asthma attack frequency.
The normal range of blood eosinophil count is 30–350 cells/μL; however, there is controversy with respect to the cut-off level associated with increased risk of asthma complications. In clinical trials involving mepolizumab, Austin et al. [34] found that the rate of clinically significant asthma exacerbations varied according to blood eosinophil level and employed blood eosinophil cut-offs of ≥ 150 to ≥ 300 cells/μL.
Severe asthma that is not controlled despite optimal treatment represents about 10% of asthma population. Presence of eosinophilic inflammation pathway in the respiratory tract and blood is involved and interleukin-5 (IL-5) has recently been identified as a major promotor of this pathway. The anti-IL-5 antibodies reduce the incidence of exacerbation and allowed steroid sparing in severe asthma patients. Anti-IL-5 antibodies are now a standard treatment for severe eosinophilic asthma that can also be useful in an emergency to treat steroid-refractory eosinophilic acute severe asthma [35].
IgE biomarker was a significant risk factor for acute severe asthma but not a predictor for asthma exacerbation severity according to this study. A recent study by Haselkorn et al. [36] on the long-term predictors of poorly controlled asthmatics demonstrated that higher total IgE levels were noted in poorly-controlled persistent asthmatics than in other asthmatics.
In this study, SO2 < 90% is considered a predictor for asthma exacerbation severity and hospitalization significantly with odds ratio value equals 4.56 and 5.67 respectively which is in concordance with previous studies.
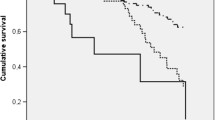
In patients with asthma, the PEFR percent predicted correlates reasonably well with the percent predicted value for the forced expiratory volume in one second (FEV1) and provides an objective measure of airflow limitation when spirometry is not available. In this current study, each patient was asked to perform PEFR on three occasions but the results showed that PEFR after 1-h treatment was a predictor for acute severe exacerbation with OR =3.34 and 95% confidence interval (1.90–4.9). PEFR values after 1 h and 6 h of treatment were reliable predictors for exacerbation severity but PEFR value on admission was not (Figs. 1 and 2).
ROC curve shows accuracy of PEFR in prediction of severe acute exacerbation in asthmatic patients. PEFR, peak expiratory flow rate. Baseline PEFR (blue line) cut-off point< 80 (sensitivity 45%, specificity 89%, accuracy 64.8%, and AUC 0.70), PEFR after 1 h (green line) cut-off point < 260 (sensitivity 100%, specificity 62%, accuracy 82.9%, and AUC 0.81), and PEFR after 6 h (orange line) cut-off point < 110 (sensitivity 65%, specificity 88.2%, accuracy 75.5%, and AUC 0.72).
Kole and colleagues stated that persistent airflow limitation (PAL) defined as a post-bronchodilator FEV1/forced vital capacity (FVC) < lower limit of normal represented 33% of severe asthma recruited patients and also in 16% of patients with milder disease. In patients with mild asthma, more caution and intense treatment should be considered as this group was associated with a higher level of eosinophilic inflammation and a higher risk of exacerbations [37].
Bloom et al.’s study [38] showed that during 7 years of follow-up, exacerbations occur in around one-third of patients. Of those who exacerbate, half did not frequently exacerbate, so the timing of future exacerbations is largely unpredictable. Just 2% exhibit a frequent-exacerbating phenotype. Past exacerbation patterns are the most informative risk factor for predicting future exacerbations [38].
Conclusion
Severe asthma exacerbations can be anticipated by determined risk factors as age >40 years, uncontrolled disease symptoms, oral steroids use, and overuse of SABA. Comorbidities as DM, GERD, OSA, food allergy, and moderate and severe depression make patients at risk for acute severe asthma exacerbation. Patients with high blood eosinophils and high Ig E levels are at risk for acute severe asthma. The most accurate independent predictor for severe asthma exacerbation is the PEFR value after 1 h of treatment.
Limitations of the study
Our study recruited a limited sample of selected patients in a single-center study; also, specific clinical characteristics of the study population (acute severe asthma) may limit the generalization of the results.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ABG:
-
Arterial blood gases
- AR:
-
Allergic rhino-sinusitis
- BAI:
-
Beck’s Anxiety Inventory
- BDI-II:
-
Beck Depression Inventory 2
- CAS:
-
Critical asthma syndromes
- ETS:
-
Environmental tobacco smoke
- ERV:
-
Expiratory reserve volume
- FEV1:
-
Forced expiratory volume in 1st second
- FVC:
-
Forced vital capacity
- FRC:
-
Functional residual capacity
- GERD:
-
Gastro-esophageal reflux disease
- ICS:
-
Inhaled corticosteroids
- IgE:
-
Immunoglobulin E
- MMAS-4:
-
Morisky Medication Adherence Scales
- PAL:
-
Persistent airflow limitation
- PEF:
-
Peak expiratory flow
- PEFR:
-
Peak expiratory flow rate
- SABA:
-
Short acting ßeta agonist
- Th2:
-
T-helper cell type 2
References
Global Initiative for Asthma (GINA) Global Strategy for Asthma Management and Prevention (2021 Update) [(accessed on 10 August 2021)]; Available online: https://ginasthma.org/wp-content/uploads/2021/05/GINA-Main-Report-2021-V2-WMS.pdf
Ramnath VR, Clark S, Camargo CA (2007) Multicenter study of clinical features of sudden-onset versus slower-onset asthma exacerbations requiring hospitalization. Respiratory care. 52(8):1013–1020
Serrano-Pariente J, Rodrigo G, Fiz JA, Crespo A, Plaza V, High Risk Asthma Research Group (2015) Identification and characterization of near-fatal asthma phenotypes by cluster analysis. Allergy. 70(9):1139–1147
Kenyon N, Zeki AA, Albertson TE, Louie S (2015) Definition of critical asthma syndromes. Clinical reviews in allergy & immunology. 48(1):1–6
Kostakou E, Kaniaris E, Filiou E, Vasileiadis I, Katsaounou P, Tzortzaki E et al (2019) Acute severe asthma in adolescent and adult patients: current perspectives on assessment and management. Journal of clinical medicine. 8(9):1283
Gaga M, Zervas E, Samitas K, Bel EH (2012) Severe asthma in adults: an orphan disease? Clinics in chest medicine. 33(3):571–583
Aron J, Akbari O (2017) Regulatory T cells and type 2 innate lymphoid cell-dependent asthma. Allergy. 72(8):1148–1155
Papi A, Brightling C, Pedersen S, Reddel H (2017) Asthma. Lancet 391(2):783–800
Global Initiative for Asthma (GINA) Global Strategy for Asthma Management and Prevention (2019 Update) [(accessed on 6 August 2019)]; Available online: https://ginasthma.org/wp-content/uploads/2019/06/GINA-2019-main-report-June-2019-wms.pdf
Morisky DE, Ang A, Krousel-Wood M, Ward HJ (2008) Predictive validity of a medication adherence measure in an outpatient setting. The journal of clinical hypertension. 10(5):348–354
Thomas M, Kay S, Pike J, Williams A, Rosenzweig J, Hillyer E et al (2009) The Asthma Control Test (ACT) as a predictor of GINA guideline-defined asthma control: analysis of a multinational cross-sectional survey. Primary Care Respiratory Journal 18(1):41–49
Steer RA, Beck AT (1997) Beck Anxiety Inventory. In: Zalaquett CP, Wood RJ (eds) Evaluating stress: a book of resources. Scarecrow Education, pp 23–40
Beck AT, Steer RA, Brown GK (1996) Manual for the beck depression inventory-II. San Antonio, TX: Psychological Corporation. 1(82):10–37
Loymans RJ, Honkoop PJ, Termeer EH, Snoeck-Stroband JB, Assendelft WJ, Schermer TR et al (2016) Identifying patients at risk for severe exacerbations of asthma: development and external validation of a multivariable prediction model. Thorax. 71(9):838–846
Kang HR, Song HJ, Nam JH, Hong SH, Yang SY, Ju S et al (2018) Risk factors of asthma exacerbation based on asthma severity: a nationwide population-based observational study in South Korea. BMJ open. 8(3):e020825
Yii ACA, Tan JHY, Lapperre TS, Chan AKW, Low SY, Ong TH et al (2017) Long term future risk of severe exacerbations: distinct 5 year trajectories of problematic asthma. Allergy 72:1398–1405
Alvarez GG, Schulzer M, Jung D, Fitzgerald JM (2005) A systematic review of risk factors associated with near-fatal and fatal asthma. Canadian respiratory journal. 12(5):265–270
Gonzalez-Barcala FJ, San-Jose ME, Nieto-Fontarigo JJ, Carreira JM, Calvo-Alvarez U, Cruz MJ, et al (2018) Association between blood eosinophil count with asthma hospital readmissions. Eur J Intern Med 53:34–9
Silverman RA, Hasegawa K, Egan DJ, Stiffler KA, Sullivan AF, Camargo CA Jr (2017) Multicenter study of cigarette smoking among adults with asthma exacerbations in the emergency department, 2011–2012. Respiratory medicine. 125(7):89–91
Merianos AL, Dixon CA, Mahabee-Gittens EM (2017) Secondhand smoke exposure, illness severity, and resource utilization in pediatric emergency department patients with respiratory illnesses. Journal of Asthma. 54(8):798–806
To M, Hitani A, Kono Y, Honda N, Kano I, Haruki K, To Y (2018) Obesity-associated severe asthma in an adult Japanese population. Respiratory investigation. 56(6):440–447
Holguin F, Bleecker ER, Busse WW, Calhoun WJ, Castro M, Erzurum SC et al (2011) Obesity and asthma: an association modified by age of asthma onset. Journal of Allergy and Clinical Immunology. 127(6):1486–1493
Sideleva O, Suratt BT, Black KE, Tharp WG, Pratley RE, Forgione P et al (2012) Obesity and asthma: an inflammatory disease of adipose tissue not the airway. American journal of respiratory and critical care medicine. 186(7):598–605
Kimura H, Konno S, Makita H, Taniguchi N, Shimizu K, Suzuki M et al (2018) Prospective predictors of exacerbation status in severe asthma over a 3-year follow-up. Clinical & Experimental Allergy. 48(9):1137–1146
Belachew SA, Erku DA, Yimenu DK, Gebresillassie BM (2018) Assessment of predictors for acute asthma attack in asthmatic patients visiting an Ethiopian hospital: are the potential factors still a threat? Asthma research and practice. 4(1):1–7
Levy M, Andrews R, Buckingham R, Evans H, Francis C, Houston R, Lowe D, Nasser S, Pation J, Puri N, Stewart K (2014) Why asthma still kills: The national review of asthma deaths (NRAD) confidential enquiry report. Royal College of Physicians.
Khattab A, Madkour A, Ambaram A, Smith C, Muhwa CJ, Mecha JO et al (2022) Over-prescription of short-acting β2-agonists is associated with poor asthma outcomes: results from the African cohort of the SABINA III study. Curr Med Res Opin. 27:1–13
Neffen H, Chahuàn M, Hernández DD, Vallejo-Perez E, Bolivar F, Sánchez MH et al (2020) Key factors associated with uncontrolled asthma–the Asthma Control in Latin America Study. Journal of Asthma. 57(2):113–122
Belachew EA, Tadess S, Alemayehu M, Ayele EM (2022) Level of asthma control and its determinants among adults living with asthma attending selected public hospitals in northwestern, Ethiopia: using an ordinal logistic regression model. Asthma Res Pract. 8(1):5
Chen E, Miller GE (2007) Stress and inflammation in exacerbations of asthma. Brain, behavior, and immunity. 21(8):993–999
Ritz T, Wittchen H-U, Klotsche J, Mühlig S, Riedel O (2016) Asthma trigger reports are associated with low quality of life, exacerbations, and emergency treatments. Annals of the American Thoracic Society. 13(2):204–211
Zeiger RS, Schatz M, Dalal AA, Chen W, Sadikova E, Suruki RY et al (2017) Blood eosinophil count and outcomes in severe uncontrolled asthma: a prospective study. The Journal of Allergy and Clinical Immunology. In Practice. 5(1):144–153
Jackson DJ, Humbert M, Hirsch I, Newbold P, Gil EG (2020) Ability of serum IgE concentration to predict exacerbation risk and benralizumab efficacy for patients with severe eosinophilic asthma. Advances in therapy. 37(2):718–729
Austin D, Pouliquen I, Yancey S, Bleeker E (2016) A blood eosinophil count of greater than 150 cells/uL predicts sputum eosinophilia≥ 3% in patients with severe asthma with other markers of inflammatory lung disease. InB101. PHENOTYPING OF ASTHMA IN THE ERA OF BIOMARKERS AND OMICS. American Thoracic Society, p A4338–A4338
Barbarot N, Nourry E, Massart N, Legay F, Debarre M, Fillatre P et al (2022) Treating acute severe eosinophilic asthma with IL-5 inhibitors in ICU. Case Rep Pulmonol. 21(2022):2180795
Haselkorn T, Szefler SJ, Chipps BE, Bleecker ER, Harkins MS, Paknis B et al (2020) Disease burden and long-term risk of persistent very poorly controlled asthma: TENOR II. The Journal of Allergy and Clinical Immunology. In Practice. 8(7):2243–2253
Kole TM, Berghe EV, Kraft M, Vonk JM, Nawijn MC, Siddiqui S, Sun K, Fabbri LM, Rabe KF, Chung KF, Nicolini G (2022) Predictors and associations of the persistent airflow limitation phenotype in asthma: a post-hoc analysis of the ATLANTIS study. Lancet Respir Med
Bloom CI, Palmer T, Feary J, Quint JK, Cullinan P (2019) Exacerbation patterns in adults with asthma in England. A population-based study. American journal of respiratory and critical care medicine. 199(4):446–453
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
AZEM, LHS, SSF: conception and design. WGE and EA: data collection. EA: statistical analysis. LHS and WGE: medical writing. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the institutional review board and ethical committee of Faculty of Medicine- Assiut University in compliance with the Helsinki Declaration (IRB: 17100097).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mohamed, A.Z.E.LA., Shaaban, L.H., Gad, S.F. et al. Acute severe asthma in emergency department: clinical characteristics, risk factors, and predictors for poor outcome. Egypt J Bronchol 16, 57 (2022). https://doi.org/10.1186/s43168-022-00160-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43168-022-00160-8