Abstract
Introduction
Asthma is a heterogenous disease with various phenotypes that is characterized by airway limitation due to bronchospasm and airway inflammation associated with excessive mucus secretion. Eosinophilic asthma subtype is described as a late onset asthma that presents with more severe respiratory symptoms, and with sputum eosinophilia ≥ 3%. In the current study, we aimed to identify the difference in the clinical and demographic characteristics between eosinophilic and non-eosinophilic asthma subtypes and to determine predictors of eosinophilic asthma.
Materials and methods
One hundred bronchial asthma patients with age ≥ 18 years were divided into two groups according to sputum eosinophilia. All patients were subjected to medical history, Asthma Control Test (ACT), spirometry, serum IgE level, skin prick testing (SPT), and nasal endoscopy to detect nasal polyposis and allergic signs.
Results
No statistical difference was found between eosinophilic and non-eosinophilic asthma patients regarding age, gender, and body mass index. Patients with sputum eosinophilia had more severe obstruction by spirometry, and positive SPT to food allergens, pollens, and latex with statistical significance (p values 0.001, 0.016, and 0.017 respectively). Additionally, patients with sputum eosinophilia had lower ACT score, higher serum IgE level and higher serum eosinophil count. Total IgE had the highest diagnostic accuracy for discrimination of sputum eosinophilia among asthma patients. Pollen allergy and the severity of airway obstruction by spirometry were independent predictors of eosinophilic asthma.
Conclusion
Patients with eosinophilic asthma had more severe airway obstruction, lower ACT scores, higher serum IgE level, and serum eosinophil count. Pollen allergy and obstructive pattern by spirometry were independent predictors of eosinophilic asthma.
Similar content being viewed by others
Introduction
Asthma is a heterogeneous disease with various phenotypes. Recent literature has focused on the need for unbiased multidimensional endotyping of asthma to adequately address the obvious disease complexity [1]. Adult-onset eosinophilic asthma is recently considered both a severe and a difficult to treat phenotype of asthma [2]. Although to date there is no exact definition of eosinophilic asthma, patients with this asthma subtype have late onset, more severe asthma, poor symptom control, frequent exacerbations, and oral corticosteroid dependence. It is estimated that around 25% of patients with severe asthma have a late-onset eosinophilic asthma subtype [3].
Eosinophilic asthma subtype presents with atypical respiratory symptoms which may be misleading and falsely diagnosed as chronic obstructive pulmonary disease (COPD) due to the presence of fixed airway obstruction [4] and frequent severe exacerbations [5]. Eosinophils are recognized as a main factor contributing to airway remodeling [6]. Eosinophilia in the sputum/blood that is driven by type 2 immune responses account for severe eosinophilic asthma subtype [7]. A cut-off of ≥ 3% of sputum eosinophils is well correlated with diagnosis of eosinophilic asthma [8]. It is of particular importance to identify the eosinophilic inflammation and to identify distinct clinical characteristics of eosinophilic asthma subtype in part due to the severity of attacks and significant steroid non-responsiveness in those particular patients. Additionally, the emergence of biological drugs, such as omalizumab and newer biological agents as anti-interleukin (IL)-5 are considered particularly effective in this subtype of asthma, thus playing a role in reducing exacerbations, significantly improving quality of life, and positively affecting the ability to discontinue chronic steroid dependence [9].
Diagnosing eosinophilic asthma subtype is challenging, and research has focused on the essential need for early and correct diagnosis. Identifying asthma endotypes is a pre-requisite to precise personalized management of asthmatic patients [10]. To our knowledge, no studies discuss asthma subtyping in Egyptian asthmatics. Hence, the current study aimed to identify the difference in the demographic and clinical characteristics between eosinophilic and non-eosinophilic asthma subtypes and to determine predictors of eosinophilic asthma.
Materials and methods
The current cross-sectional comparative study recruited 100 bronchial asthma patients diagnosed according to GINA 2020 guidelines [11] with age ≥ 18 years selected by cluster randomization from the allergy outpatient clinic of Ain Shams University Hospitals. All asthma patients recruited were receiving regular treatment with medium to high dose inhaled corticosteroids therapy, with or without oral corticosteroids. Patients were divided into two groups according to the results of sputum eosinophilia into patients with sputum eosinophilia (number = 49) and patients without sputum eosinophilia (number = 51). All patients gave informed written consent for participating in the study, and the study was carried in accordance with the Declarations of Helsinki. Ethical approval was obtained from the Ethical Committee of Ain Shams University Hospitals.
Smokers, patients with COPD, patients with concomitant infections or parasitic infestations, and those who refused to give verbal consent, were excluded from the study.
All patients were subjected to detailed history taking including age, gender, occupation, age of onset of asthma, documented allergy, and family history of allergic diseases. Assessment of asthma severity was done according to the Global Initiative for Asthma (GINA) criteria 2020 [11].
Asthma Control Test (ACT)
The ACT questionnaire is a validated self-administered questionnaire including five questions related to the last 4 weeks: episodes of breathlessness, nocturnal awakenings, limitations of daily activities, need for rescue medication, and patient’s self-rating of asthma control. Each question includes five response modalities with a score ranging from one to five by increasing level of asthma control [12].
Skin prick test (SPT)
SPT was done according to Bernstein et al. 2008 [13]. Twenty six of the common locally encountered allergens were used for SPT, as well as a positive histamine control and a negative saline control on the forearm. The test was considered positive if the wheal was greater than 3 mm in diameter.
Allergens used for SPT were food allergens (milk, eggs, fish, wheat, nuts, peanut, soya, and shellfish), pollens (trees, grasses and weeds), animal allergens (cat dander, dog hair, pigeon feather and rabbit hair), house dust mites (Dermatophagodies pteronyssinus, Deramatophagoides farinae), cockroach, moulds (Aspergillus fumigatus, Cladosporium, and Alternaria), and latex.
Sputum eosinophilia
Specimen collection
Patients were provided with an explanation of the specimen required, pointing out the difference between saliva and sputum. They were instructed to sit up in an upright position on a chair or on the edge of the bed (high Fowler position) to ensure maximum lung expansion. The patient’s mouth was rinsed with water before the sample was collected, to avoid contaminating the sample with food residues. It was ensured that patients are well hydrated to help increase sputum production. Ten minutes’ sodium chloride (0.9%) nebulizer was prescribed to help loosen secretions. The patient took several deep breaths (breathing in through the nose and exhaling though the mouth; to help loosen secretions) before giving a deep cough to release the sputum, then expectoration into a sterile leak proof container was done. The sample was transported to the microbiology lab of Ain Shams University Hospitals for processing.
Sputum processing
The sputum sample was homogenized by adding equal amount of phosphate-buffered saline (PBS) in a 10-ml sterile test tube, and centrifuged for 10 min. 0.1% dithiothreitol (Sigma Chemicals, UK) was added to the sample in a ratio of 4:1, which was agitated for 20 min to break up the disulfide bonds and disperse the cells. The sample was then placed in a shaking water bath at 37 °C for 15 min to ensure complete homogenization. To stop the effect of DTT, the suspension was further diluted with PBS to twice the volume equal to the sputum plus DTT. The cell suspension was filtered through a 120-lm mesh Nylon Net Filter (Filter NY: 2H00010-Millipore, France) to remove debris and mucus. Supernatant was separated from cell pellet. The sample was transferred to the slide and was distributed thinly and evenly over the slide.
Sputum staining and eosinophil detection
Staining was done by leishmain stain and analyzed using ordinary light microscopy (high power magnification) starting at the top left corner in an undulating manner from top to bottom while moving across the slide to detect eosinophils. The eosinophil count was then expressed as a percentage, i.e., four hundred non-squamous cells were counted, and the results were expressed as a percentage of the total non-squamous count. The eosinophil count was then expressed as a percentage.
Sputum eosinophil count ≥ 3% was considered abnormal. Eosinophil count was expressed as a percentage as it is more accurate than absolute count [14].
Total IgE
Serum IgE level was measured by ELISA using Elecsys IgE II reagent kit in cobas e411 analyzers.
Spirometry
The degree of reversibility in forced expiratory volume in one second (FEV1) of 12% or 200 ml from the pre-bronchodilator value was considered as diagnostic for asthma as per GINA guideline.
Nasal endoscopy
The rigid endoscope allows for detailed examination of the nasal cavity. The endoscope can be rotated laterally under the middle turbinate into the posterior aspect of the middle meatus. An excellent view of the middle turbinate, uncinate process, and surrounding mucosa was obtained to accurately detect allergic signs (violaceous nasal mucosa and hypertrophied nasal turbinates) and nasal polyposis.
Statistical methods
Data were analyzed using IBM© SPSS© Statistics version 23 (IBM© Corp., Armonk, NY) and MedCalc© version 18.2.1 (MedCalc© Software bvba, Ostend, Belgium).
Discrete or skewed numerical data were presented as median and interquartile range and between-group differences were compared using the Mann-Whitney U test. Categorical variables were presented as number and percentage and differences were compared using the Pearson chi-squared test. Ordinal data were compared using the chi-squared test for trend. Correlations were tested using the Spearman rank correlation. Receiver operating characteristic (ROC) curve analysis was used to examine the diagnostic value of blood eosinophil count or total serum IgE for diagnosis of eosinophilic asthma. Multivariable binary logistic regression analysis was used to examine predictors of eosinophilic asthma. Two-sided p values < 0.05 were considered statistically significant.
Results
Basic demographic and clinical characteristics of all patients are displayed in Table 1. Mean ± SD age of participants was 35.4 ± 12.8 years, and age of asthma onset was 3.95 ± 3.4 years.
Table 2 shows comparison of patients with or without sputum eosinophilia. ACT denoted poorly- controlled asthma in patients with sputum eosinophilia with statistical significance. Additionally, patients with sputum eosinophilia had positive SPT to food allergens, pollens and latex at a higher frequency than patients with negative sputum eosinophilia with statistical significance (p value = 0.001, 0.016, and 0.017, respectively).
Regarding numerical variables, Table 3 shows that patients with sputum eosinophilia had lower ACT scores, higher absolute serum eosinophil count, and higher serum IgE level with statistical significance (p value < 0.0001, 0001, and < 00001 respectively).
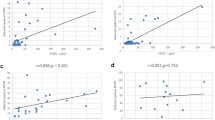
Figure 1 displays receiver operating characteristic (ROC) curve analysis for discrimination between patients with eosinophilic and non-eosinophilic asthma using the ACT score, blood eosinophil count, and total serum IgE level.
Total serum IgE level has a good diagnostic value (area under ROC curve = 0.816, P value < 0.001). The cut-off criterion of total serum IgE level > 80 IU/ml (sensitivity = 71%, specificity = 84%) has a positive predictive value and negative predictive value of 81.4% and 75.4% respectively.
The ACT score has a fair diagnostic value for discrimination between patients with eosinophilic and non-eosinophilic asthma (area under ROC curve = 0.779, P value < 0.001). The best cut-off criterion for discrimination is ACT score ≤ 18 (sensitivity = 67%, specificity = 78%), with a positive predictive value and negative predictive value of 75% and 71.4% respectively.
Serum eosinophil count has a poor diagnostic value (area under ROC curve = 0.686, P value < 0.001). The best cut-off criterion is serum eosinophil count > 0.12 × 103/μL (sensitivity = 86%, specificity = 49%), with a positive predictive value and negative predictive value of 61.8% and 78.1% respectively.
Table 4 shows the results of backward logistic regression analysis for predictors of eosinophilic asthma. Pollen allergy (odds ratio = 5.399, 95% CI = 1.594 to 18.284, P value = 0.007) and presence of a more severe obstructive pattern by spirometry (odds ratio = 8.807, 95% CI = 2.989 to 25.953, P value < 0.001) were independent predictors of eosinophilic asthma.
Discussion
To our knowledge, the current prospective study is the first to compare eosinophilic and non-eosinophilic asthma subtypes among Egyptian patients with bronchial asthma. In clinical practice, routine measurement of sputum eosinophils is hindered by technical and practical considerations, however in research context, sputum eosinophils of 3% or higher is used to identify eosinophilic asthma subtype [15].
We found no statistically significant difference between eosinophilic and non-eosinophilic asthma patients regarding age, gender, body mass index (BMI) and age of onset of asthma. Despite that eosinophilic asthma is documented to present at a later stage of life than non-eosinophilic asthma [16], some studies have detected no significant difference in age [17] and gender [18] between both asthma subtypes. Additionally, authors have suggested that eosinophilia can be detected in multiple asthma phenotypes, eosinophilic inflammation is a characteristic of and a diagnostic biomarker for both childhood-onset allergic asthma and later-onset asthma [19].
Eosinophilic asthma patients had statistically significantly higher frequency of positive SPT to food allergens, pollens, and latex allergens. Higher sputum eosinophils were detected in children with asthma and food allergies [20], which is in line with the results of SPT performed in our study population.
Eosinophilic asthma patients showed more severe airway obstruction by spirometry and positive allergic signs by nasal endoscopy with statistical significance. It is reported that late-onset eosinophilic asthma is associated with chronic sinusitis and nasal polyps [9]. Patients with eosinophilic asthma in the current study showed a higher frequency of nasal polyposis, yet the difference was non-significant statistically.
Regarding asthma control, asthma was poorly controlled in patients with positive sputum eosinophilia with statistical significance. Additionally, serum eosinophil absolute count and serum IgE level were statistically significantly higher in those patients. Lee et al. found that eosinophilic asthmatics had moderate to severe asthma, higher serum eosinophil count, and lower forced expiratory volume in 1 s (FEV1), which is in line with our findings despite their study enrolled children not adults [17]. There was significant correlation between serum IgE level and eosinophil count in another study in children [21].
In the current study, serum IgE level at a cut-off > 80 IU/ml showed 71% sensitivity and 84% specificity for discriminating between eosinophilic and non-eosinophilic asthma patients, representing a good diagnostic value in comparison to ACT score and serum eosinophil count which had fair and poor diagnostic values respectively. Additionally, positive predictive value and negative predictive value of total IgE in discriminating eosinophilic from non-eosinophilic asthma was 81.4% and 75.4% respectively.
Although total IgE has long been described as the classical biomarker in asthma [22], some authors conversely found serum eosinophils had a superior diagnostic accuracy in diagnosing patients with airway eosinophilia, and combining fractional exhaled nitric oxide (FeNO) and serum eosinophils further improved the overall diagnostic accuracy [23].
Nonetheless, in the present study, a cut-off serum eosinophil count > 0.12 × 103/μL discriminated eosinophilic and non-eosinophilic asthma with poor diagnostic accuracy. There is conflicting data regarding serum eosinophil count. Normal peripheral eosinophil count did not exclude airway eosinophilic asthma in children with severe therapy resistant asthma [24]. Nonetheless, it is hypothesized that blood eosinophil count of less than 90 eosinophils per microliter are highly unlikely to have airway eosinophilia. A higher cut-off value of ≥ 0.41 × 103/μL (absolute count) was associated with sputum eosinophils ≥ 3% in 95% of patients [23]. Additionally, baseline blood eosinophils ≥ 0.15 × 103/μL was selected by the DREAM study as a predictive biomarker for response to Mepolizumab in patients with severe asthma and eosinophilic inflammation [25]. However, blood eosinophil count as a predictive biomarker does not necessarily reflect levels if used as a diagnostic biomarker. Additionally, the use of biologics does not require investigation for sputum eosinophilic inflammation [23].
Regarding the results of SPT in patients with eosinophilic asthma, we found that pollen allergy by SPT and the severity of obstruction by spirometry both represented independent predictors of eosinophilic asthma.
Conclusion
To conclude, we found no difference in age and gender between eosinophilic and non-eosinophilic asthma patients. Eosinophilic asthma patients had poorly controlled asthma and positive allergic signs by nasal endoscopy. Serum IgE with a cut-off > 80 IU/ml showed 71% sensitivity and 84% specificity for discriminating between eosinophilic and non-eosinophilic asthma patients, with a better diagnostic value than ACT score and serum eosinophil count. Independent predictors of asthma were positive SPT to pollen allergens and more sever obstruction by spirometry.
Among the limitations of the current study is being a single center study with a small sample size. Documenting serum markers of eosinophil degranulation as eosinophilic cationic protein [25] could further validate the current study’s results. We also recommend objective evaluation of airway obstruction using biomarkers such as serum periostin and FeNO to identify patients with eosinophilic asthma in future studies. Eosinophilic asthma patients should be referred to an ENT specialist for evaluation of allergic signs and nasal polyposis that may contribute to asthma severity and poor disease control, and future studies should focus on treatment options for this relatively severe asthma subtype.
Abbreviations
- ACT:
-
Asthma Control Test
- SPT:
-
Skin prick test
- BMI:
-
Body mass index
- ECP:
-
Eosinophilic cationic protein
References
Agache I (2019) Severe asthma phenotypes and endotypes. Semin Immunol. 46:101301
Durrington HJ, Gioan-Tavernier GO, Maidstone RJ, Krakowiak K, Loudon ASI, Blaikley JF, Fowler SJ, Singh D, Simpson A, Ray DW (2018) Time of day affects eosinophil biomarkers in asthma: implications for diagnosis and treatment. Am J Respir Crit Care Med 198:1578–1581
Miranda C, Busacker A, Balzar S, Trudeau J, Wenzel SE (2004) Distinguishing severe asthma phenotypes: role of age at onset and eosinophilic inflammation. J Allergy Clin Immunol. 113:101–108
Peters MC, Kerr S, Dunican EM, Woodruff PG, Fajt ML, Levy BD, Israel E, Phllips BR, Mauger DT, Comhair SA et al (2019) National Heart, Lung and Blood Institute Severe Asthma Research Program 3. Refractory airway type 2 inflammation in a large subgroup of asthmatic patients treated with inhaled corticosteroids. J Allergy Clin Immunol. 143(1):104–113.e14
Hasegawa K, Stoll SJ, Ahn J, Bittner JC, Camargo CA (2015) Prevalence of Eosinophilia in Hospitalized Patients with Asthma Exacerbation Respiratory Medicine 109(9):1230–32
Bakakos A, Loukides S, Bakakos P (2019) Severe eosinophilic asthma. J. Clin Med. 8(9):1375
Choi Y, Sim S, Park HS (2020) Distinct functions of eosinophils in severe asthma with type 2 phenotype: clinical implications. Korean J Intern Med. 35(4):823–833
Khatry DB, Gossage DL, Geba GP, Parker JM, Jarjour NN, Busse WW, Molfino NA (2015) Discriminating sputum-eosinophilic asthma: accuracy of cutoffs in blood eosinophil measurements versus a composite index, ELEN. J Allergy Clin Immunol. 136:812–814 e2
Rogliani P, Calzetta L, Matera MG, Laitano R, Ritondo BL, Hanania NA, Cazzola M (2020) Asthma and biological therapy: when, which, and for whom. Pulm Ther 6:47–66
Kaur R, Chupp G (2019) Phenotypes and endotypes of adult asthma: Moving toward precision medicine. J Allergy Clin Immunol. 144(1):1–12
“Global Strategy for Asthma Management and Prevention, Global Initiative for Asthma (GINA),” 2020. http://www.ginasthma.org/. [Accessed 8 Oct, 2020].
Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, Murray JJ, Pendergraft TB (2004) Development of the asthma control test: a survey for assessing asthma control. J. Allergy Clin. Immunol. 113:59–65
Bernstein IL, Li JT, Bernstein DI, Hamilton R, Spector SL, Tan R, Sicherer S, Golden DBK, Khan DA, Nicklas RA et al (2008) Allergy diagnostic testing: an updated practice parameter. Ann Allergy Asthma Immunol 100(3):S1 148
Kumar RM, Pajanivel R, Koteeswaran G, Menon SK, Charles PMV (2017) Correlation of total serum immunoglobulin E level, sputum, and peripheral eosinophil count in assessing the clinical severity in bronchial asthma. Lung India 34(3):256–261
Arron JR, Choy DF, Scheerens H, Matthews JG (2013) Noninvasive biomarkers that predict treatment benefit from biologic therapies in asthma. Ann Am Thorac Soc. 10:S206–S213
Aleman F, Lim HF, Nair P (2016) Eosinophilic endotype of asthma. Immunol Allergy Clin N Am. 36:559–568
Lee YJ, Kim KW, Choi BS, Sohn MH, Kim KE (2013) Clinical characteristics of eosinophilic and noneosinophilic asthma in children. Acta Paediatr. 102(1):53–57
van Veen IH, Ten Brinke A, Gauw SA, Sterk PJ, Rabe KF, Bel EH (2009) Consistency of sputum eosinophilia in difficult-to-treat asthma: a 5-year follow-up study. J Allergy Clin Immunol 124:615–617
Carr TF, Kraft M (2018) Use of biomarkers to identify phenotypes and endotypes of severe asthma. Ann Allergy Asthma Immunol. 121(4):414–420
Kulkarni N, Ragazzo V, Costella S, Piacentini G, Boner A, O’Callaghan C, Fiocchi A, Kantar A (2012) Eosinophilic airway inflammation is increased in children with asthma and food allergies. Pediatr Allergy Immunol. 23:28–33
Lababidi HM, AlSowayigh OM, BinHowemel SF, AlReshaid KM, Alotaiq SA, Bahnassay AA (2019) Refractory asthma phenotyping based on immunoglobulin E levels and eosinophilic counts: a real life study. Respir Med. 158:55–58. https://doi.org/10.1016/j.rmed.2019.10.003
Szefler SJ, Wenzel S, Brown R, Erzurum SC, Fahy JV, Hamilton RG, Hunt JF, Kita H, Liu AH, Panettieri RA Jr, Schleimer RP, Minnicozzi M (2012) Asthma outcomes: biomarkers. J Allergy Clin Immunol. 129(3 Suppl):S9 23
Westerhof GA, Korevaar DA, Amelink M, de Nijs SB, de Groot JC, Wang J, Weersink EJ, ten Brinke A, Bossuyt PM, Bel EH (2015; In press DOI) Biomarkers to identify sputum eosinophilia in different adult asthma phenotypes. Eur Respir J. https://doi.org/10.1183/09031936.00012415
Ullmann N, Bossley CJ, Fleming L, Silvestri M, Bush A, Saglani S (2013) Blood eosinophil counts rarely reflect airway eosinophilia in children with severe asthma. Allergy. 68(3):402–406
Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON, Ortega H, Chanez P (2012) Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 380:651–659
Acknowledgements
We would like to thank Dr. Marwa Elashry, associate professor of Clinical Pathology for advising the sputum analysis technique. We thank Dr. Marwa Mohammed El-Begermy, associate professor of Otorhinolaryngology for aiding in the nasal endoscopy procedure (the answer for the presence of an ENT dr).
We would also like to thank all the patients who participated in the study.
Funding
Nil
Author information
Authors and Affiliations
Contributions
Maged Refaat shared in conception of the study design and revised the work for important intellectual content. Dina Sheha wrote the manuscript and supervised data collection. Riham Raafat shared in data collection and analysis and revised the work. Heba Abualia performed the research, collected the data and analyzed the data, and edited the manuscript. All authors read and approved the final version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Refaat, M.M., Raafat, R.H., AbuAlia, H.E. et al. Identifying clinical and demographic characteristic differences between eosinophilic and non-eosinophilic asthma and detecting predictors of eosinophilic asthma among Egyptian asthmatic patients. Egypt J Bronchol 16, 53 (2022). https://doi.org/10.1186/s43168-022-00157-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43168-022-00157-3