Abstract
Background
Bronchopleural fistula (BPF) is a sinus tract between the pleural space and the main stem, lobar, or segmental bronchus. The development of a bronchopleural fistula (BPF) is associated with high rates of morbidity and mortality. An interdisciplinary approach, early diagnosis, and timely management of these lesions are critical in the management of such lesions.
Case presentation
We describe a case of bronchopleural fistula in a 42-year-old female patient, occurring after a surgery for pulmonary hydatid, which was successfully managed using a minimally invasive method of closure using Amplatzer vascular plug (AVP).
To our knowledge, the use of an AVP for the management of a BPF following hydatid cyst marsupialization has rarely been described in the past.
Conclusion
AVP is a useful device in the management of bronchopleural fistulas, especially in patients failing a trial of conservative management and are high-risk candidates for surgeries.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Background
BPF, a sinus tract between bronchi and the pleural space, occurs as a complication following thoracic surgeries such as lobectomy, thoracotomy, and pulmonary infection [1]. BPFs are associated with high rates of morbidity and mortality with reported mortality ranging as high as 70% [2, 3].
Treatment for BPF ranges from conservative/medical management to bronchoscopic procedures for critically ill patients and surgical intervention for those at the highest risk. There is no consensus as to the best available treatment for the management of such fistulae.
The various surgical options include thoracostomy, thoracoplasty, and direct closure of the fistula using flaps of different origins [4]. Surgery generally carries a dismal prognosis in patients with a poor general condition. Moreover, the recurrence rate of BPF following a surgical repair could be as high as 23.6% [4]. The recurrence rate of BPF following a surgical repair carries a mortality greater than 50%, which is caused by respiratory insufficiency and uncontrolled sepsis [5].
Bronchoscopic management of BPFs is based on the delivery of N- butyl cyanoacrylate glue, coils, emphysema valves, autologous blood patch, AVP, occlusive material to the fistula [1], and tracheobronchial stents [6].
Out of the abovementioned methods, coils, glue, and emphysema valves are preferred for smaller BPFs and are a poor choice for larger fistulas. Larger fistulas provide a poor framework for these occlusive materials and can lead to inadvertent migration of the occlusive material into the pleural space [7,8,9].
Blood patches have been used for the management of BPFs with poor outcome rates ranging from 27-72%. The shortcomings of blood patch for the management of BPFs include the risk of pleural infection. Also, complete closure of fistulas using blood patches required multiple bronchoscopy sessions with closure rates following the first session being as low as 56% [10].
Management of BPF using different types of stents has been described. Stents can however lead to sputum retention by impeding mucociliary clearance [7]. Also, stents were not available at our institution at the time of this case.
Amplatzer plugs have been used for the management of congenital septal defects [11], pulmonary arteriovenous malformations, anomalous venous connections, and internal iliac arteries [12, 13].
AVPs have been successfully used for the management of BPFs with no recurrent disease in almost all previously described studies (Table 1).
AVP are a good choice for BPFs which are long and wide such as the one in this case.
AVP devices are made of two discs with a central waist made of a mesh of braided nitinol. The central waist measures from 4 to 40 mm in diameter. The distal disc is about 14mm and the proximal disc is 10 mm larger, respectively, than the diameter of the central waist. This provides an anchoring lip of about 5–7 mm circumferentially.
The waist is positioned inside the defect, while the discs anchor the device on either side of the fistula. AVP devices can be collapsed into low-profile delivery sheaths (5–7 French) owing to the superelastic and shape memory properties of nitinol. This allows the advancement of the devices across the lesion [8].
The plug is attached to a 155-cm-long delivery wire with a micro screw made of stainless steel, allowing the operator to release the plug into the final position by rotating the cable using a supplied torque device. The plug can be readjusted and retrieved as needed before it is finally released. The AVP is compatible with magnetic resonance imaging (MRI), within a magnetic field of less than 3 Tesla [23].
They are designed to provide immediate effective closure of the defect, are available in a large range of sizes, and can be appropriately matched to the lesion. The presence of two disks, one on either side of the lesion, leads to greater coverage and increases the likelihood of closure, as opposed to glue, which is applied to the internal aspect alone.
The devices induce endothelial response and potentiating granulation tissue without causing airway compromise which eventually leads to closure of the fistula [13, 24].
Case presentation
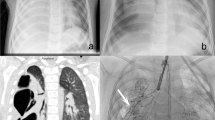
A 42-year-old female, a case of pulmonary hydatidosis (Fig. 1), planned for video-assisted thoracoscopic marsupialization of hydatid cyst in the left lung on 23/07/2021. An intercostal drainage (ICD) tube was kept in the operative bed.
The patient was recuperating well until day 4 after surgery when bubbling was noted through the ICD tube. This progressed to subcutaneous emphysema spreading to the neck and showed worsening progression (Fig. 2).
Computed tomography (CT) scan of the thorax showed a fistulous communication, measuring about 1cm wide and 6cm long, between the left pleural cavity and segmental bronchus for the left lower lobe. This was suggestive of a bronchopleural fistula (Fig. 3).
A Non-contrast axial chest CT and B minimum intensity projection (MinIP) in the coronal plane showing the fistulous communication (white arrows) between the post-operative cavity (C) and the segmental bronchus for the lower lobe. Also, a note is made of the extensive subcutaneous emphysema (yellow arrows) and ICD tube in situ
As the fistula was wide and long, closure of the bronchopleural fistula using the Amplatzer vascular plug-2 (AGA Medical, Golden Valley, Minn., USA) device was planned. The patient was taken for the procedure under fluoroscopy and bronchoscopy guidance. The patient was intubated maintaining ventilation to both the lungs while the closure procedure was carried out on the fistula in the left lung.
Technique
A 9mm Amplatzer vascular plug-2 (AVP) was used for the closure of the fistula. The size of the vascular plug chosen was according to the diameter of the bronchial stump and the length of the fistula so that the device would completely cover the fistulous connection fitting within the stump. The delivery cable was introduced through the endotracheal tube under constant visualization by fluoroscopy. Once positioned within the fistula the plug was released. After its deployment, a bronchogram was performed by injecting a contrast medium through a catheter to check the position of the plug and ensure exclusion of the fistula (Fig. 4).
Follow-up CT and X-rays confirmed the closure of the fistulous communication (Fig. 5).
Conclusion
The endobronchial approach for the management of BPF is effective especially in patients failing a trial of conservative management and high-risk candidates for surgeries. AVP is a useful device in the armamentarium of endobronchial management of big fistulae, which cannot be managed using coils, glues, stents, or blood patches.
Availability of data and materials
The data and materials supporting the findings of this study are available on request from the corresponding author.
Abbreviations
- AVP:
-
Amplatzer vascular plug
- BPF:
-
Bronchopleural fistula
- CT scan:
-
Computed tomography scan
- ICD:
-
Intercostal drainage tube
- MRI:
-
Magnetic resonance imaging
References
Salik I, Vashisht R, Abramowicz AE (2021) Bronchopleural fistula. [Updated 2021 May 12]. In: StatPearls. StatPearls Publishing, Treasure Island
Karfis EA, Kakadellis J (2009) Video-thoracoscopic management of a postpneumonectomy bronchopleural fistula. Gen Thorac Cardiovasc Surg 57:675–677
Farkas EA, Detterbeck FC (2006) Airway complications after pulmonary resection. Thorac Surg Clin 16(3):243–251
Bribriesco A, Patterson GA (2018) Management of postpneumonectomy bronchopleural fistula: from thoracoplasty to transsternal closure. Thorac Surg Clin 28(3):323–335
de la Riviere AB, Defauw JJ, Knaepen PJ, van Swieten HA, Vanderschueren RC, van den Bosch JM (1997) Transsternal closure of bronchopleural fistula after pneumonectomy. Ann Thorac Surg 64(4):954–957 discussion 958–959
Zeng J, Wu X, Chen Z et al (2021) Modified silicone stent for the treatment of post-surgical bronchopleural fistula: a clinical observation of 17 cases. BMC Pulm Med 21:10. https://doi.org/10.1186/s12890-020-01372-8
Chae EY, Shin JH, Song HY, Kim JH, Shim TS, Kim DK (2010) Bronchopleural fistula treated with a silicone-covered bronchial occlusion stent. Ann Thorac Surg 89(1):293–296. https://doi.org/10.1016/j.athoracsur.2009.05.068
Fruchter O, El Raouf BA, Abdel-Rahman N, Saute M, Bruckheimer E, Kramer MR (2014) Efficacy of bronchoscopic closure of a bronchopleural fistula with amplatzer devices: long-term follow-up. Respiration 87(3):227–233. https://doi.org/10.1159/000357074
Ferguson JS, Sprenger K, Van Natta T (2006) Closure of a bronchopleural fistula using bronchoscopic placement of an endobronchial valve designed for the treatment of emphysema. Chest 129(2):479–481. https://doi.org/10.1378/chest.129.2.479
Thapa B, Sapkota R, Sayami P (2017) Autologous blood patching in the management of broncho-pleural fistula in spontaneous pneumothorax. J Soc Surg Nepal 18(2):23–28. https://doi.org/10.3126/jssn.v18i2.18571
Barwad P, Ramakrishnan S, Kothari SS, Saxena A, Gupta SK, Juneja R, Gulati GS, Jagia P, Sharma S (2013) Amplatzer vascular plugs in congenital cardiovascular malformations. Ann Pediatr Cardiol 6(2):132–140. https://doi.org/10.4103/0974-2069.115255
Hart JL, Aldin Z, Braude P, Shovlin CL, Jackson J (2013) Embolization of pulmonary arteriovenous malformations using the Amplatzer vascular plug: successful treatment of 69 consecutive patients. Eur Radiol 20(11):2663–2670. https://doi.org/10.1007/s00330-010-1851-2
Cil B, Peynircioğlu B, Canyiğit M, Geyik S, Ciftçi T (2008) Peripheral vascular applications of the Amplatzer vascular plug. Diagn Interv Radiol 14(1):35–39
Kramer MR, Peled N, Shitrit D, Atar E, Saute M, Shlomi D, Amital A, Bruckheimer E (2008) Use of Amplatzer device for endobronchial closure of bronchopleural fistulas. Chest 133(6):1481–1484. https://doi.org/10.1378/chest.07-1961
Scordamaglio PR, Tedde ML, Minamoto H, Pedra CA, Jatene FB (2009) Endoscopic treatment of tracheobronchial tree fistulas using atrial septal defect occluders: preliminary results. J Bras Pneumol 35(11):1156–1160. English, Portuguese. https://doi.org/10.1590/s1806-37132009001100015
Gulkarov I, Paul S, Altorki NK, Lee PC (2009) Use of Amplatzer device for endobronchial closure of bronchopleural fistulas. Interact Cardiovasc Thorac Surg 9(5):901–902. https://doi.org/10.1510/icvts.2009.215202
Fruchter O, Bruckheimer E, Raviv Y, Rosengarten D, Saute M, Kramer MR (2012) Endobronchial closure of bronchopleural fistulas with Amplatzer vascular plug. Eur J Cardiothorac Surg 41(1):46–49. https://doi.org/10.1016/j.ejcts.2011.02.080
Tedde ML, Scordamaglio PR, Rodrigues A, Minamoto H, Alfinito FS (2011) Minimally invasive closure of bronchopleural fistulas. Chest 140(3):826
Yang L, Kong J, Tao W, Song Y, Huang T, He F, Zhang P, Cai X, Dou Y, Wang Z (2013) Tuberculosis bronchopleural fistula treated with atrial septal defect occluder. Ann Thorac Surg 96(1):e9–e11
Marwah V, Rajput AK, Madan H, Garg Y (2014) Closure of chronic bronchopleural fistula using atrial septal occluder device. J Bronchology Interv Pulmonol 21(1):82–84. https://doi.org/10.1097/LBR.0000000000000034
Klotz LV, Gesierich W, Schott-Hildebrand S, Hatz RA, Lindner M (2015) Endobronchial closure of bronchopleural fistula using Amplatzer device. J Thorac Dis 7(8):1478–1482. https://doi.org/10.3978/j.issn.2072-1439.2015.08.25
Scordamaglio PR, Tedde ML, Minamoto H, Assad RS, Fernandes PMP (2017) Can total bronchopleural fistulas from complete stump dehiscence be endoscopically treated? Eur J Cardiothorac Surg 51(4):702–708. https://doi.org/10.1093/ejcts/ezw377
Lopera JE (2015) The Amplatzer vascular plug: review of evolution and current applications. Semin Intervent Radiol 32(4):356–369. https://doi.org/10.1055/s-0035-1564810
Han YM, Gu X, Titus JL, Rickers C, Bass JL, Urness M, Amplatz K (1999) New self-expanding patent foramen ovale occlusion device. Catheter Cardiovasc Interv 47(3):370–376. https://doi.org/10.1002/(SICI)1522-726X(199907)47:3<370::AID-CCD27>3.0.CO;2-9
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
AG conceived of the study, analyzed and performed the procedure, participated in the manuscript design, and helped in drafting the manuscript; SD drafted the manuscript and participated in manuscript design and coordination. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the institutional ethical committee. Written informed consent was obtained from the patient for the publication of this case report and accompanying images.
Name of the ethics committee approving the study- Institutional Ethics Committee, Department of Pharmacology, Grant Government Medical College & Sir J.J. Group of Hospitals, Mumbai-400008.
Consent for publication
All authors read and approved the final manuscript. The patient included in this case gave written informed consent to publish the data and materials contained within this study.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gutte, A.A., Dembla, S. Endobronchial management of bronchopleural fistula using vascular plug device—a case report. Egypt J Bronchol 16, 50 (2022). https://doi.org/10.1186/s43168-022-00152-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43168-022-00152-8