Abstract
Until now, there are more than two hundred million confirmed cases of COVID-19 including more than seven million deaths. Clinical trials of all three vaccines authorized for use in the UK (Pfizer–BioNTech, Oxford–AstraZeneca, and Moderna) have reported high vaccine efficacy. This rapid systematic review was initiated because no systematic review had been conducted to determine the safety and efficacy of AstraZeneca ChAdOx1 nCoV-19 vaccine. Evidence acquisition: A systematic search in the following platforms: PubMed, Google Scholar, Scopus, WOS, and MEDLINE databases for all articles in the English language regarding safety and efficacy of SARS-CoV-2 vaccine ChAdOx1 nCoV-19 was performed. Papers published up to end of June were included. Evidence synthesis: Out of 477 retrieved articles, fifteen are included. All the selected articles are concerned with evaluation of AstraZeneca ChAdOx1 nCoV-19 vaccine. Three of them discussed the effectiveness of ChAdOx1 nCoV-19 vaccine, while thirteen (one is common with the group of the effectiveness) measured the adverse effects associated with the vaccine. Because thrombosis was recorded as a serious adverse effect developed after ChAdOx1 nCoV-19 vaccination, it was emphasized in a special group to be analyzed separately. In conclusion: the main message of selected papers was that the value of ChAdOx1 nCoV-19 vaccination to provide critical protection should be considered higher compared to the significant worldwide burden of the emerging COVID-19 infection. No causal relations were found to link cases- having thrombotic adverse reactions to the vaccine.
Similar content being viewed by others
Introduction
In early December 2019, the Chinese Center of Disease Control reported that SARS-CoV-2 infection is the cause of the outbreak that started in Wuhan City [1]. SARS-CoV-2 virus is the third member of coronaviruses that causes epidemics in human history following SARS-COV and MERS. It is highly infectious and can spread globally and rapidly [2]. Until now, there are more than two hundred million confirmed cases of COVID-19 including more than seven million deaths [3].
Vaccines mimic the virus—or part of the virus—so they can protect against stimulation of the immune system to produce antibodies. Their safety standards must be higher than other medicines as they are used for the prevention of infectious diseases in healthy people and reduction of morbidity and mortality without long-lasting effects [4, 5].
For that reason, scientists are in a race with time to discover new vaccines against COVID-19. There are more than 170 candidate vaccines that are now being followed up by the World Health Organization (WHO) [6]. The first COVID-19 vaccines were approved shortly after the initial phase 3 safety and efficacy studies [7]. Clinical trials of all three vaccines authorized for use in the UK (Pfizer–BioNTech, Oxford–AstraZeneca, and Moderna) have reported high vaccine efficacy [8,9,10].
Large post-licensing epidemiological studies are needed to complement the results of pre-licensing trials to estimate the efficacy of these vaccines at the population level in real-world conditions, because vaccine development normally takes a very long period to confirm that vaccines are safe and effective before they are used.
This rapid systematic review was initiated because no systematic review had been conducted to determine the safety and efficacy of AstraZeneca ChAdOx1 nCoV-19 vaccine especially after publishing a number of case series which revealed serious adverse effects associated with the vaccine such as life-threatening thrombocytopenic thrombosis.
Methods
Study design
The study was designed as a systematic review according to PRISMA guidelines [11]. All steps of this study were pre-specified, and the protocol was registered on Clinicaltrial.gov: NCT05060861.
Search strategy
On May 22, 2021, we searched PubMed, Google Scholar, Scopus, WOS, and MEDLINE databases for all articles in English regarding the safety and efficacy of the SARS-CoV-2 vaccine ChAdOx1 nCoV-19. The search strategy can be retrieved in supplementary digital material 1. Materials available as gray literature were followed and searched in pre-print platforms (MedRxiv, BoiRxiv), protocols, WHO reports, conference posters, thesis, or trial registers in ClinicalTrial.gov.
Study selection
Two authors (I.A.M and M.A) independently completed all searches and removed all duplicate records. We selected the articles based on titles and abstracts. The second and last screening stage was performed by two authors (I.A.M and M.A), and discrepancies and doubts were solved by a consensus with two more authors (R.S, I.H.I). We critically appraised the full text of each study that was included if respected one of the following inclusion criteria: (1) P: volunteers (aged 18 years old or more), (2) I: ChAdOx1 nCoV-19 vaccine, (3) C: any comparator vaccine 4) O: Efficacy and Safety, (5) study design: Randomized controlled trials, retrospective studies, cohort, case-control, case series, survey, and recommendation, (6) Language: only English
Data extraction
A data extraction form was created in word. Data were extracted by three authors (R.S., I.A.M. and M.A.) comprising the following data (if applicable): (1) study name (author/year), (2) study design, (3) study period, (4) setting (institute, city, and country), (5) study protocol number, (6) aim of study, (7) main and secondary outcome, (8) target population, (9) main age of study population, (10) classification of population according to gender, (11) sample size, (12) dose, (13) method of evaluation, and (14) conclusion
Risk of bias assessment
We did not appraise the quality of included studies due to urgency and need of rapid appraisal of published data in this topic.
Statistical analyses
The statistical analysis was performed using open meta-analyst software [12,13,14]. Dichotomous and continuous data were pooled as untransformed proportion (PR) and standardized mean difference (SMD), respectively, in a random-effects model with 95% confidence interval (CI). Heterogeneity was assessed by observation of the graphs on forest plots and measured by chi-square test and I-square tests for the degree of the heterogeneity. Between studies, significant heterogeneity was defined as a chi-square test with p<0.1 and I2 tests >50% [15]. We considered the endpoints statistically significant with p value <0.05. Irrespective of the between-study heterogeneity, subgroup analysis was done for all efficacy endpoints based on the method by which the efficacy was measured in the included studies, and the adverse events were measured depending on the number of cases developed these adverse events after vaccination.
Results and evidence synthesis
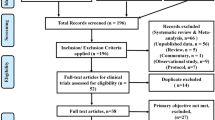
Out of 477 retrieved articles, fifteen are included [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30]. Figure 1 provides all details about the study selection process. All the selected articles are concerned with the evaluation of the AstraZeneca ChAdOx1 nCoV-19 vaccine. Three of them are concerned with the effectiveness of the ChAdOx1 nCoV-19 vaccine [18, 23, 29], while thirteen (one is common with the group of the effectiveness) consider the adverse effects associated with the vaccine [16, 17, 19,20,21,22,23,24,25,26,27,28, 30]. Because thrombosis is a serious adverse effect developed after ChAdOx1 nCoV-19 vaccination, it was placed in a special group to be analyzed separately [16, 20, 24,25,26,27,28, 30] (Table 1).
Efficacy outcomes
Three studies reported the effectiveness of the ChAdOx1 nCoV-19 vaccine. A total of 1,078,284 persons received the 1st dose and responded to the effectiveness evaluation so they are included in the analysis. The overall effect size significantly favored the effectiveness of the vaccine.
-
Two studies evaluated the effectiveness by decreasing SARS CoV-2 positive tests after vaccination, in which 458,130 received the 1st dose and responded to effectiveness evaluation so included in analysis. ChAdOx1 nCoV-19 vaccine significantly decreased the positive SARS-CoV-2 tests, 291806 of 458130 had negative test results after vaccination (PR= 0.675, 95% CI [0.528, 0.822], P < 0.001). The pooled studies were heterogeneous (chi-square p<0.001, I2=99.99%) (Fig. 2).
-
Two studies (one study is common between 2 groups) evaluated the effectiveness by decreasing the hospitalization, in which 965,434 received the 1st dose and responded to effectiveness evaluation so included in the analysis. ChAdOx1 nCoV-19 vaccine significantly decreased hospital admission, 752,904 of 965,434 were not hospitalized after the vaccination (PR= 0.74, 95% CI [0.466, 1.014], P < 0.001), the pooled studies were heterogeneous (chi-square p<0.001, I2=99.99%) (Fig. 3).
-
Two studies evaluated the effectiveness in elderly. In which 965,434 received the 1st dose and responded to effectiveness evaluation so included in the analysis. ChAdOx1 nCoV-19 vaccine significant in elderly, 756,357 of 965,434 had -ve SARS-CoV-2 test results and were not hospitalized after vaccination (PR= 0.745, 95% CI [0.480, 1.010], P < 0.001), the pooled studies were heterogeneous (chi-square p<0.001, I2= 99.999%) (Fig. 4).
Safety outcomes
Seven studies reported the safety of the ChAdOx1 nCoV-19 vaccine. A total of 635,109 persons received the 1st dose and responded to safety evaluation so included in analysis. Of them 427,613 were female (PR=0.683, 95% CI [0.569, 0.797], P < 0.001). The pooled studies were heterogeneous (chi-square p<0.001, I2 = 99.983%).
-
Analysis showed that 123,969 of 353,302 have more than one side effect, (PR=0.717, 95% CI [0.339, 1.094], P<0.001), the pooled studies were heterogeneous (chi-square p<0.001, I2 = 99.99%).
-
A total number of 86,811 of older population -who were vaccinated- showed at least one side effects (PR=0.439, 95% CI [0.245, 0.633], P<0.001), the pooled studies were heterogeneous (chi-square p<0.001, I2 = 99.997%).
-
Also, 36,191 of younger population—who were vaccinated—showed at least one side effects (PR=0.579, 95% CI [0.143, 1.014], P<0.001), the pooled studies were heterogeneous (chi-square p<0.001, I2 = 99.998%).
-
Malaise (75.20%), headache (23.86%), fatigue (22.39%), vomiting (21.06%), chills (15.80%), joint pain (12.30%), fever (9.08%), muscle pain (8.48%), nausea (5.84%), diarrhea (2.58%), and bleeding (0.02%) are the most reported systemic side effects of ChAdOx1 nCoV-19 vaccine (Table 2).
-
Local pain (11.53%), itching (2.48%), swelling (3.07%), redness (2.41%), and skin rash (0.50%) are the most reported local side effects of ChAdOx1 nCoV-19 vaccine (Table 2).
-
Death was reported in only 18 of 281,272 among the vaccinated population, this is insignificant value, (PR=0.148, 95% CI [− 0.211, 0.508], P =0.418), the pooled studies were heterogeneous (chi-square p <0.028, I2= 79.161%). figures is in supplementary material 2
Thrombosis outcomes
Eight studies reported thrombosis adverse events of the ChAdOx1 nCoV-19 vaccine. 281347 received the 1st dose of AstraZeneca vaccine and responded to thrombosis adverse events evaluation so included in analysis. Two hundred twenty-two thousand twenty-six of them are female (PR=0.784, 95% CI [0.755, 0.814], P < 0.001). The pooled studies were homogeneous (chi-square p = 0.404, I2= 3.415%).
-
Standardized mean difference of age in the cases of thrombotic adverse events = 41.519 years old (95% CI [36.352, 46.686], p< 0.001), platelet count = 39.873×109/L (95% CI [27.387, 52.359], p< 0.001), aPTT Activated partial thromboplastin time = 29.943 s, (95% CI [25.406, 34.481], p< 0.001), INR peak = 1.271 (95% CI [1.152, 1.391], p< 0.001), fibrinogen = 1.444 g/l, (95% CI [1.015, 1.872], p< 0.001), and D-dimer = 33.047 mg/l (95% CI [22.703, 43.392], p< 0.001). The pooled studies were heterogeneous (chi-square p ≤ 0.001, I2= 90.204%, 95.818%, 95.724%, 85.277%, 88.658%, and 93.423%, respectively).
-
The studies recorded 33 cases—of total 17,132,686 vaccinated—having thrombotic adverse reactions and this is an insignificant value (PR=0, 95% CI [− 0.000, 0.000], P = 0.371). The pooled studies were heterogeneous (chi-square p = 0.033, I2= 78.123%).
-
Thirty-one cases showing more than one thrombotic adverse reaction - of total 33 who had thrombotic adverse reaction, (PR= 0.515, 95% CI [0.281, 0.749], P < 0.001). The pooled studies were heterogeneous (chi-square p < 0.001, I2= 84.009%).
-
The most reported thrombotic adverse events are deep venous thrombosis (77 cases), thrombosis in other organs/areas (57 cases), cerebral venous sinus thrombosis (46 cases), pulmonary embolism (23 cases), and splanchnic vein thrombosis (23 cases) (Table 2).
-
Twenty- nine of 281,334 is the number of deaths in the studies that reported thrombotic adverse reactions (PR= 0.132, 95% CI [0.008, 0.257], P < 0.001). The pooled studies were heterogeneous (chi-square p ≤ 0.001, I2= 79.289%). Figures are in supplementary material 3
Discussion
This investigation involved a systematic review and meta-analysis of RCTs, cohorts, case series, case reports, case-control, and cross-sectional studies to summarize the efficacy and safety of the ChAdOx1 nCoV-19 vaccine. This investigation comprised 9 cohorts, 2 case reports, 1 RCTs, 1 case series, 1 case-control, and 1 cross-sectional study with a total sample size of 1,368,188 patients, 107,8284 of them were analyzed to evaluate the efficacy, and 635,184 were analyzed to evaluate the safety, with 345,280 common between two groups.
The study findings revealed that the first doses of the ChAdOx1 vaccines were associated with protection against COVID-19 admission to hospital and a decrease in the number of positive cases among the vaccinated population. A vaccine effect of 78% for protection against hospitalization and 63.7% for decreasing in the number of +ve cases among the vaccinated population. In the elderly age group, based on a pooled analysis for the vaccine, we observed vaccine efficiency of 78.3%.
The most reported systemic adverse effects associated with ChAdOx1 vaccine are malaise (75.20%), headache (23.86%), fatigue (22.39%), vomiting (21.06%), chills (15.80%), joint pain (12.30%), fever (9.08%), muscle pain (8.48%), nausea (5.84%), diarrhea (2.58%), and bleeding (0.02%). The percentage of older people with at least one adverse event (22.7%) is larger than the percentage of younger people with at least one adverse event (21.13%).
Cases with thrombotic adverse events had mean platelet count = 39.873 × 109/L, (lower than normal mean) and Activated partial thromboplastin time (aPTT) = 29.943 s (within normal value). Despite these values, thrombosis also occurred. However, the recorded INR peak was 1.271, this value is lower than normal range and this may stimulate thrombosis formation. The fibrinogen level was1.444 g/l, and D-dimer 33.047 mg/l. The most reported thrombotic adverse events were deep venous thrombosis (77 cases), thrombosis in other organs/areas (57 cases), cerebral venous sinus thrombosis (46 cases), pulmonary embolism (23 cases), and splanchnic vein thrombosis (23 cases).
The increasing number of reports on rare thrombotic events after SARS-CoV-2 vaccination draw public attention and led to concerns regarding the safety of this vaccine due to the uncertainty of the origin of these undesired reactions
The limitations of this study include the small number of potential thrombotic adverse events which were contained from case reports and case series but it shouldn't be neglected because these are serious adverse events that lead to death. The quality of the included studies was not evaluated to decide the importance of the included data, due to lack of time during the pandemic.
However, this study has provided valuable information about the safety and efficacy of the ChAdOx1 vaccine from trusted databases with a large sample size and summarizes all the literature which is published until the time of searching.
Finally, the observed clinical and laboratory features of the VITT are exceptional and rare and the reported side effects cannot lead to death mostly and are relieved by medical treatment except in a few cases. Therefore, the value of COVID-19 vaccination to provide critical protection should be considered higher compared to the significant health risk of COVID-19. With the better recognition of this rare complication and the availability of efficient therapies, the risk-benefit ratio of ChAdOx1 nCoV-19 might be reconsidered further.
Conclusions
All selected articles are based on published literature about the viral vector COVID-19 vaccine “ChAdOx1 nCoV-19.” The main message is that the value of COVID-19 vaccination ChAdOx1 nCoV-19 to provide critical protection should be considered higher compared to the significant health risk of COVID-19. Further updates are needed to follow the emerging vaccines and recognize their safety and efficacy against different variants of the novel virus
Availability of data and materials
Data are available in the supplementary file.
References
Lu H, Stratton CW, Tang YW (2020) Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracle. J Med Virol 92(4):401
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y et al (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395(10223):497–506
Coronavirus W (2021) Dashboard| WHO Coronavirus (COVID-19) Dashboard with Vaccination Data
André FE (2001) The future of vaccines, immunization concepts and practice. Vaccine 19(17-19):2206–2209
Zhang C, Maruggi G, Shan H, Li J (2019) Advances in mRNA vaccines for infectious diseases. Front Immunol 10:594
Kommenda N, Hulley-Jones F (2020) Covid vaccine tracker: when will a coronavirus vaccine be ready? The Guardian
Ramasamy MN, Minassian AM, Ewer KJ, Flaxman AL, Folegatti PM, Owens DR et al (2020) Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet 396(10267):1979–1993
Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Marc GP, Moreira ED, Zerbini C, Bailey R (2020) Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. New England J Med
Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK et al (2021) Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 397(10269):99–111
Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R et al (2021) Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. New England J Med 384(5):403–416
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097
Lau J, Antman EM, Jimenez-Silva J, Kupelnick B, Mosteller F, Chalmers TC (1992) Cumulative meta-analysis of therapeutic trials for myocardial infarction. New England J Med 327(4):248–254
Viechtbauer W (2010) Conducting meta-analyses in R with the metafor package. J Stat Software 36(3):1–48
Wallace BC, Dahabreh IJ, Trikalinos TA, Lau J, Trow P, Schmid CH (2012) Closing the gap between methodologists and end-users: R as a computational back-end. J Stat Software 49(1):1–15
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560
Althaus K, Möller P, Uzun G, Singh A, Beck A, Bettag M et al (2021) Antibody-mediated procoagulant platelets in SARS-CoV-2-vaccination associated immune thrombotic thrombocytopenia. Haematologica 106(8):2170
Bae S, Lee YW, Lim SY, Lee J-H, Lim JS, Lee S et al (2021) Adverse reactions following the first dose of ChAdOx1 nCoV-19 vaccine and BNT162b2 vaccine for healthcare workers in South Korea. J Korean Med Sci 36(17):e115
Bernal JL, Andrews N, Gower C, Robertson C, Stowe J, Tessier E et al (2021) Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ 373:n1088
Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S et al (2020) Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 396(10249):467–478
Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S (2021) Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. New England J Med 384(22):2092–2101
Jeon M, Kim J, Oh CE, Lee J-Y (2021) Adverse events following immunization associated with coronavirus disease 2019 vaccination reported in the mobile vaccine adverse events reporting system. J Korean Med Sci 36(17):e114
Kim S-H, Wi YM, Yun SY, Ryu JS, Shin JM, Lee EH et al (2021) Adverse events in healthcare workers after the first dose of ChAdOx1 nCoV-19 or BNT162b2 mRNA COVID-19 vaccination: a single center experience. J Korean Med Sci 36(14):e107
Menni C, Klaser K, May A, Polidori L, Capdevila J, Louca P et al (2021) Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect Dis
Pottegård A, Lund LC, Karlstad Ø, Dahl J, Andersen M, Hallas J et al (2021) Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: population based cohort study. BMJ 373:n1114
Schultz NH, Sørvoll IH, Michelsen AE, Munthe LA, Lund-Johansen F, Ahlen MT et al (2021) Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. New England J Med 384(22):2124–2130
Scully M, Singh D, Lown R, Poles A, Solomon T, Levi M et al (2021) Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. New England J Med 384(23):2202–2211
Tiede A, Sachs UJ, Czwalinna A, Werwitzke S, Bikker R, Krauss JK et al (2021) Prothrombotic immune thrombocytopenia after COVID-19 vaccine. Blood 38:350–353
Tobaiqy M, Elkout H, MacLure K (2021) Analysis of Thrombotic Adverse Reactions of COVID-19 AstraZeneca Vaccine Reported to EudraVigilance Database. Vaccines 9(4):393
Vasileiou E, Simpson CR, Shi T, Kerr S, Agrawal U, Akbari A et al (2021) Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: a national prospective cohort study. Lancet 397(10285):1646–1657
Wolf ME, Luz B, Niehaus L, Bhogal P, Bäzner H, Henkes H (2021) Thrombocytopenia and intracranial venous sinus thrombosis after “COVID-19 vaccine AstraZeneca” exposure. J Clin Med 10(8):1599
Acknowledgements
None
Funding
The authors did not receive funds for the accomplishment of this study.
Author information
Authors and Affiliations
Contributions
A AA Mohamed Hussein was the team leader, responsible for checking the validity of the study, follow up all steps, writing, and editing; I Hussein, I Mahmoud, and M Amary were responsible for database searching, examination of records, full-text assessment; R Alsayad was the student leader, examined eligible studies, and performed the qualitative synthesis. All authors have read and approved the final version of the study.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the ethical committee Assist Faculty of Medicine.
Consent for publication
N/A.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rationale: This systematic review was initiated because no systematic review had been conducted to determine the safety and efficacy of AstraZeneca ChAdOx1 nCoV-19 vaccine especially after publishing a number or case series which revealed serious adverse effects associated with the vaccine such as Thrombocytopenia.
Supplementary Information
Additional file 1: Supplementary 1.
Modified search strategy in different databases https://docs.google.com/document/d/17NEtXgr_giWpiyVnHGdyqqs5UNZS5zGytXAQhhlgF7w/edit?usp=sharingSupplementary 2. Folder containing forest plots of adverse events analysis https://drive.google.com/drive/folders/17mv5gFSiRbiH6gZN16qeN_8Iix8RqmlS?usp=sharingSupplementary 3. Folder containing forest plots of thrombotic adverse events analysis https://drive.google.com/drive/folders/1mFN6bv2Y0atfHl0OeZKU4EmhLWFkY1Nq?usp=sharing .
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mohamed Hussein, A.A.R., Ibrahim, I.H., Mahmoud, I.A. et al. To what extent AstraZeneca ChAdOx1 nCoV-19 vaccine is safe and effective? Rapid systematic review. Egypt J Bronchol 16, 6 (2022). https://doi.org/10.1186/s43168-021-00109-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43168-021-00109-3