Abstract
Background
Lupus mesenteric vasculitis (LMV) is a serious complication of systemic lupus erythematosus (SLE) that can lead to a range of gastrointestinal issues, such as intestinal bleeding, necrosis, and perforation. The optimal surgical approach for cases of intestinal necrosis in LMV remains a topic of debate.
Case presentation
We present the first documented instance of jejunal necrosis in a 56-year-old woman undergoing SLE treatment. The patient presented with symptoms including abdominal distension, pain, nausea, and vomiting. Physical examination revealed abdominal tenderness, rebound pain, and muscle tension. Following surgery and steroid therapy, the patient experienced successful recovery and was discharged from the hospital.
Conclusions
Patients with jejunal necrosis due to SEL, during intraoperative exploration, surgical decisions should be made based on careful observation. In cases of extensive jejunal necrosis that cannot be preserved, it is advisable to resect the necrotic tissue as much as possible, with a recommended extended resection margin of 2–3 cm.
Similar content being viewed by others
Background
Systemic lupus erythematosus (SLE) is a complex autoimmune disease that can impact any part of the body, leading to a variety of symptoms [1]. One such symptom is lupus vasculitis (LV), which manifests differently based on the specific blood vessels and organs or tissues affected [2]. When LV affects the mesenteric vessels, it is known as lupus mesenteric vasculitis (LMV), characterized by abdominal symptoms like pain, distension, nausea, and vomiting. Physical examination often shows tenderness and signs of peritoneal irritation. While numerous studies have demonstrated positive outcomes with early immunosuppression and high-dose steroid therapy for LMV [3], there remains a debate regarding the optimal surgical approach in cases where intestinal necrosis has developed in patients.
We collected data from a patient with jejunal necrosis caused by SLE, who was treated in the gastrointestinal surgery department of our hospital in July 2023. This study aims to provide guidance to surgical colleagues in the treatment of similar patients by understanding the occurrence, diagnosis, and treatment of the disease in this particular patient.
Case report
A 56-year-old female was admitted to the Department of Rheumatology and Immunology of our hospital due to “repeated facial erythema for 10 months.” Since the discovery of facial erythema, there has been no systematic examination and treatment. Hints of auxiliary examination after admission:
Laboratory inspection items: blood routine | ||
|---|---|---|
Item | Result | Normal range |
Hemoglobin concentration (HGB) | 90 g/L | 115 ~ 150 g/L |
Total protein (TP) | 56.4 g/L | 65.0 ~ 85.0 g/L |
Albumin (ALB) | 26.8 g/L | 34.0 ~ 48.0 g/L |
White blood cell (WBC) | 2.77 × 109/L | 3.50 ~ 9.50 × 109/L |
Laboratory inspection items: erythrocyte sedimentation rate | ||
|---|---|---|
Item | Result | Normal range |
Erythrocyte sedimentation rate (ESR) | 36 mm/h | 0 ~ 20 mm/h |
Laboratory inspection items: coagulation function | ||
|---|---|---|
Item | Result | Normal range |
D-dimer | 1.48 μg/ML | 0 ~ 0.55 μg/ML |
Laboratory inspection items: complement 3 (C3), complement C4 | ||
Item | Result | Normal range |
C3 | 0.26 g/L | 0.90 ~ 1.70 g/L |
C4 | 0.03 g/L | 0.12 ~ 0.36 g/L |
Laboratory inspection items: lymphocyte subsets | ||
|---|---|---|
Item | Result | Normal range |
T cell receptor complex (TCR-CD3) | 430.98/μL | 955.00 ~ 2860.00/μL |
CD4 + Helper T cells CD4(Th) | 224.94/μL | 550.00 ~ 1440.00/μL |
Cytotoxic T lymphocytes CD8 (Tc + Ts) | 181.76/μL | 320.00 ~ 1250.00/μL |
Laboratory inspection items: autoantibody | ||
|---|---|---|
Item | Result | Normal range |
Anti-double-stranded DNA antibody (dsDNA) | > 800.00 IU/ML | 0 ~ 10.00 IU/ML |
Anti-RNP/Sm (nRNP/Sm) | Positive (+ +) | Negative |
Anti-Smith antibodies (anti-Sm) | Strong positive (+ + +) | Negative |
60 kDa SS-A/Ro ribonucleoprotein antibody (SSA/60kd) | Positive (+ +) | Negative |
Antinuclear antibody (ANA) | Strong positive (+ + +) | Negative |
Anti-histone antibodies (AHA) | Weak positive (+ −) | Negative |
Anti-ribosomal P protein antibody9(ARPA/Rib-P) | Strong positive (+ + +) | Negative |
Laboratory inspection items: lupus anticoagulant | ||
|---|---|---|
Item | Result | Normal range |
β2-microglobulin | 14.54 mg/L | 1.00 ~ 3.00 mg/L |
Anti-cardiolipin antibody IgA (ACL-A) | < 2.00 RU/mL | 0 ~ 20.00 RU/mL |
Anti-cardiolipin antibody IgG (ACL-G) | 4.02 RU/mL | 0 ~ 20.00 RU/mL |
Anti-cardiolipin antibody IgM (ACL-M) | 6.94 RU/mL | 0 ~ 20.00 RU/mL |
Anti-cardiolipin antibody IgP (ACL-P) | 6.18 RU/mL | 0 ~ 20.00 RU/mL |
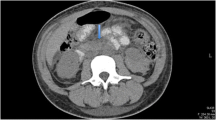
The patient was diagnosed with systemic lupus erythematosus (SLE) and had severe activity according to the SLE Disease Activity Index (SLEDAI): 15 points. Upon admission, the patient received methylprednisolone 60 mg intravenous drip once daily for anti-inflammatory and immune suppression, low molecular weight heparin calcium 4000 IU intradermal once daily for anticoagulation, proton pump inhibitor for gastric protection, and other treatments. On the 5th day of admission, the patient reported persistent and unbearable abdominal pain for 10 h. After consulting with our department, a physical examination revealed a flat abdomen with no gastrointestinal type or peristaltic wave observed, no varicose veins or abdominal skin bleeding observed, tenderness and rebound pain detected throughout the abdomen, no increase in abdominal wall muscle tension observed, and intestinal sounds auscultated at a rate of 4 times per minute. Abdominal CT examination showed significant swelling of the small intestine wall (Fig. 1a). Abdominal CTA showed filling of the mesenteric vessels (Fig. 1b).
a CT scan revealed thickening of the jejunal wall at multiple locations in the upper abdomen, with a slightly denser outer layer, exhibiting ‘target sign’ changes, along with mesenteric edema. b CTA showed the mesenteric vessels filled and displaying a ‘comb sign’ change. c During laparoscopic observation, a significant amount of turbid-brown fluid was observed in the abdominal cavity, with the portion of the bowel covered by the greater omentum appearing black. d Laparotomy revealed that the jejunal intestine, located approximately 10 cm from the Treitz’s ligament, was black and lacked peristalsis, spanning approximately 30 cm in length
Treatment
The patient presented with indications for surgery, leading us to decide on surgical intervention. During intraoperative laparoscopy, we observed a significant amount of brown turbid fluid in the abdominal cavity, along with a small amount of hemorrhagic fluid. Additionally, a portion of the intestine below the greater omentum appeared black (Fig. 1c). When we separated the omentum, we noticed slight distortion in the local mesentery below Treitz’s ligament, and the jejunum exhibited a black coloration spanning approximately 30 cm in length (Fig. 1d). The distal bowel showed slight congestion, but had good gloss and peristalsis. Consequently, we opted for a necroenterectomy. The necrotic intestine and mesentery were excised approximately 2–3 cm away from the distal and proximal ends of the necrotic intestine. Following the surgery, the patient experienced unstable vital signs and was subsequently transferred to the intensive care unit (ICU) for further treatment. The postoperative pathological findings confirmed intestinal necrosis caused by vasculitis (Fig. 2). A follow-up abdominal CT examination conducted on the 14th day after the surgery revealed no abnormalities (Fig. 3). As a result of successful treatment, the patient was discharged from the hospital.
a The pathological findings revealed chronic inflammation in the intestinal mucosa, along with interstitial hyperemia and edema. Hemorrhage was observed in the muscle layer and serosal layer of the intestinal wall, accompanied by significant infiltration of inflammatory cells. Ulcer formation was also observed in the peripheral intestinal wall (HE, 4 × 10 times). b Increased vascular wall thickness, hyalinosis, and infiltration of inflammatory cells were seen, along with polyposis at the site of vascular inflammation. Additionally, there was an overflow of red blood cells from the blood vessels (HE, 10 × 10 times)
Discussion
Cases of systemic lupus erythematosus (SLE) combined with jejunal necrosis are extremely rare worldwide. The main clinical manifestations include abdominal pain, nausea, vomiting, diarrhea, abdominal distension, and abdominal tenderness. Laboratory tests have shown that patients with lupus mesenteric vasculitis (LMV) exhibit an acceleration in erythrocyte sedimentation rate (ESR), a decrease in C3 concentration, positive dsDNA, and positive SSA [3, 4]. Abdominal CT and CTA imaging have revealed intestinal dilation, intestinal wall thickening, intestinal 'target signs’, mesenteric vascular filling, and mesenteric vascular ‘comb signs’ [3, 5].
The diagnosis of LMV primarily relies on abdominal CT and CTA. Abdominal CT findings typically include intestinal dilation, intestinal wall thickening, and intestinal tube “target signs,” while abdominal CTA findings usually involve mesenteric vascular filling and mesenteric vascular “comb signs” [3]. Comparing our cases with imaging data from LMV patients who did not develop intestinal necrosis, we found that they showed similar features. Therefore, it is believed that abdominal CT and CTA cannot effectively distinguish whether LMV patients develop intestinal ischemic necrosis. Furthermore, studies have indicated that the mortality rate of patients with intestinal ischemia lasting over 6 h exceeds 50% [6]. As a result, abdominal CT and CTA should be promptly conducted in SLE patients experiencing abdominal pain, and laparoscopic exploration should be performed if necessary to determine the presence of intestinal tube necrosis and enable early detection and treatment.
According to studies [7], high-dose steroid treatment has been found to be effective in managing abdominal symptoms in lupus patients and preventing serious complications such as intestinal ischemia, perforation, and necrosis. However, in cases of intestinal necrosis, surgical intervention becomes necessary. Indications for surgery include perforation, significant blood loss, medication failure, and signs of peritoneal inflammation. It is important to note that the risks associated with surgery are higher in lupus patients due to the presence of lupus-associated vasculitis. Incomplete removal of dead intestinal tissue during surgery can lead to complications such as intestinal leakage, abdominal infection, and sepsis. However, it is worth mentioning that surgical intervention is rarely required in SLE patients for the removal of necrotic intestinal tissue, as reported in the literature. Therefore, the optimal surgical approach for these cases remains a topic of debate. In a study conducted by Kazuma Iwasaki and colleagues [8], necrotizing ileotomy and ileostomy were performed on patients with ileal necrosis caused by antiphospholipid syndrome (APS), followed by postoperative steroid treatment. This approach yielded positive outcomes. Conversely, Grant Hubbard [9] and colleagues reported a case of severe jejunal ischemia where 3 cm of intestinal ischemic necrosis appeared at the edge of the intestinal anastomosis 24 h after resection of the ischemic necrotic intestine. The necrotic region was successfully removed through a second operation, resulting in favorable results. In our case, because the jejunum was not completely affected and the blood supply of the two stump was good, the resection area was only expanded by 2 ~ 3 cm, and then jejunostomy was performed. The patient recovered after symptomatic treatment and steroid therapy. Unfortunately, the patient died of a cerebral hemorrhage on the 8th day after discharge.
Conclusion
Jejunal necrosis due to systemic lupus erythematosus (SLE) is rare. These patients often present with non-specific abdominal symptoms, making diagnosis challenging. Abdominal CT and CTA can effectively diagnose lupus abdominal vasculitis, but cannot determine whether intestinal tube necrosis occurs. Therefore, when SLE patients experience abdominal symptoms, it is important to consider the possibility of lupus abdominal vasculitis and related acute abdominal complications such as intestinal obstruction, bleeding, perforation, and necrosis. In such cases, laparoscopic exploration should be performed to promptly detect and treat intestinal necrosis. It should be noted that lupus vasculitis can affect any organ with a rich blood supply. Therefore, during intraoperative exploration, surgical decisions should be made based on careful observation. In cases of extensive jejunal necrosis that cannot be preserved, it is advisable to resect the necrotic tissue as much as possible, with a recommended extended resection margin of 2–3 cm.
Availability of data and materials
We guarantee that the data in the article is supported by the original data. The referenced data can all be found in the PubMed database. All materials and data are available upon reasonable request.
Abbreviations
- APS:
-
Antiphospholipid syndrome
- ESR:
-
Erythrocyte sedimentation rate
- ICU:
-
Intensive care unit vasculitis
- LV:
-
Lupus vasculitis
- LMV:
-
Lupus mesenteric vasculitis
- SLEDAI:
-
SLE Disease activity index
- SLE:
-
Systemic lupus erythematosus
References
Fanouriakis A, Tziolos N, Bertsias G, Boumpas DT (2021) Update οn the diagnosis and management of systemic lupus erythematosus. Ann Rheum Dis 80(1):14–25
Calle-Botero E, Abril A (2020) Lupus Vasculitis. Curr Rheumatol Rep 22(10):71
Leone P, Prete M, Malerba E et al (2021) Lupus vasculitis: an overview Biomedicines 9(11):1626
Chen SY, Xu JH, Shuai ZW et al (2009) A clinical analysis 30 cases of lupus mesenteric vasculitis. Zhonghua Nei Ke Za Zhi 48(2):136–139
Das D, Basu K, Sarkar A (2022) Enteritis unravelled with “target sign” of lupus. Postgrad Med J 98(e1):e57–e58
Bala M, Catena F, Kashuk J et al (2022) Acute mesenteric ischemia: updated guidelines of the World Society of Emergency Surgery. World J Emerg Surg 17(1):54
Yuan S, Ye Y, Chen D et al (2014) Lupus mesenteric vasculitis: clinical features and associated factors for the recurrence and prognosis of disease. Semin Arthritis Rheum 43(6):759–766
Wang QY, Ye XH, Ding J, Wu XK (2015) Segmental small bowel necrosis associated with antiphospholipid syndrome: a case report. World J Gastroenterol 21(13):4096–4100
Hubbard G, Nerad R, Wojtasik L (2021) Hypermagnesaemia causing mesenteric ischaemia and small bowel infarction. BMJ Case Rep. 14(6)
Acknowledgements
We would like to thank the patient and his family for agreeing to publish this case. Thanks to YQL and JL for successfully completing the operation and collecting the data, and thanks to YQL for guiding the writing of this article. Thanks to SYZ and FA for collecting the data. I would also like to thank the editors for any suggestions they may have on the article.
Funding
The author is self-funded.
Author information
Authors and Affiliations
Contributions
DRF collected the data and wrote the article. YQL and JL completed the surgery and collected the data. SYZ and FA collected the data. All reviewed the article and agreed to publish it.
Corresponding author
Ethics declarations
Ethics approval and consent to participate.
The patient has signed informed written consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fu, D., Liu, Y., Zhou, S. et al. Systemic lupus erythematosus causes necrosis of the jejunum: a case report. Egypt Rheumatol Rehabil 51, 17 (2024). https://doi.org/10.1186/s43166-024-00249-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43166-024-00249-6