Abstract
Background
In SLE patients, cytokines are linked to endothelial cell damage. Nailfold capillaroscopy (NFC) is a simple method for evaluating micro-vascular abnormalities in different connective tissue diseases (CTDs). The study aimed to detect the levels of interleukin 17A (IL 17A), type I interferons (IFNs I) in the serum, and NFC changes in Egyptian SLE patients compared to a control group and to correlate NFC findings with patients’ demographic features and serum levels of IL 17A and IFNs I.
Results
Serum levels of IL 17A, IFN α, and IFN β were significantly higher in SLE patients than in control group (P < 0.0001). About thirty nine patients (73.6%) of the 53 SLE patients showed abnormal NFC changes. Egyptian SLE patients had a high prevalence of the NFC non-specific pattern, with 32 (60.4%) patients showing non-specific changes and 7 (13.2%) patients showing scleroderma pattern, including 3 (5.6%) patients with active scleroderma pattern and 4 (7.55%) patients with late scleroderma pattern. Furthermore, Raynaud’s phenomenon (RP) was observed in 8 (15.1%) SLE patients, with 3 (5.6%) having normal NFC pattern and 5 (9.4%) having scleroderma pattern. All controls (n = 20) showed normal hairpin shape capillaries. Except for SLEDAI (P = 0.03) and the presence of RP (P < 0.0001), there were no significant differences in demographic and laboratory parameters between the three NFC patterns (normal, non-specific, and scleroderma); additionally, NFC score correlated significantly with SLEDAI (P = 0.021).
Conclusion
As a result of the high disease activity, Egyptian SLE patients had elevated serum levels of IL 17A and IFNs I. The most common NFC pattern in Egyptian SLE patients was a non-specific pattern. NFC abnormalities in Egyptian SLE patients were correlated with disease activity but not with patients’ ages, disease duration, or serum levels of IL 17A and IFNs I. SLE patients with scleroderma NFC pattern and RP should be closely followed for the possibility of appearance of anti-U1 RNP antibodies and MCTDS.
Similar content being viewed by others
Background
Systemic lupus erythematosus (SLE) is an inflammatory autoimmune disease with a wide range of clinical symptoms and a chronic course with flares and remissions. The pathophysiology of SLE is significantly influenced by the activation of endothelial cells, as well as immunological abnormalities [1]. Endothelial cells produce a variety of substances that regulate vascular tone, the immune system, and the coagulation system; they are also a goal for cytokines produced during the inflammatory process [2]. Vascular injury has been considered as the origin of tissue damage in SLE pathogenesis [1].
IL 17A and IFNs I, including IFN α and IFN β, are cytokines that damage endothelial cells [3, 4]. Many studies in SLE patients [4,5,6,7] found high serum levels of these cytokines. Recent studies suggests that IL 17A is associated with SLE pathogenesis and also has a crucial role in initiating angiogenesis by stimulating vascular endothelial growth factor (VEGF), an essential factor in the process of new vessels formation [8, 9]. IFN-α and β have anti-proliferative and anti-angiogenic activity [10], and their high levels reduce levels of endothelial progenitor cells (EPCs) [3], which are important in endothelial repair and contribute to endothelial dysfunction.
Nailfold capillaroscopy (NFC) is a highly effective, low-cost, and simple imaging method used in the morphological analysis of capillaries in the nailfold area [11], with the added benefit of detecting micro-vascular changes early in some inflammatory CTDs. While NFC has been widely utilized for diagnosing systemic sclerosis and scleroderma-like diseases [12], there is a growing body of evidence suggesting its potential utility in detecting early microangiopathic changes in SLE [13]. Moreover, recent studies have indicated a correlation between microcapillary pathological alterations and the clinical progression of SLE [13].
Few studies have been conducted on the correlation between serum cytokine levels and microvascular abnormalities observed by NFC in SLE patients. Therefore, the study aimed to detect the levels of IL 17A, IFNs I in the serum, and NFC changes in Egyptian SLE patients compared to a control group and to correlate NFC findings with patients' demographic features, IL 17A and IFNs I levels.
Patients and methods
Fifty-three SLE patients (46 women and 7 men, mean age 32.5 ± 10.6 years) were recruited for the study, according to the updated 1982 American College of Rheumatology (ACR) criteria for SLE [14], from the out-patient clinics of immunology and rheumatology department from January 2021 to August 2022. The local ethics committee approved the study (D-1–2022) and all participants received informed consent before the beginning of the study. Patients with any of the following diseases were excluded from the study: diabetes mellitus, uncontrolled hypertension, overlap syndrome, malignancy, and chronic infections. On the same day of collecting blood samples, the physicians applied the physical examination for the patients and the rheumatologist performed NFC measurements for all subjects. The patients were also evaluated for the existence of Raynaud’s phenomenon (RP). The systemic lupus erythematosus diseases activity index (SLEDAI) was used for the assessment of disease activity [15] with a maximum score of 105 points and a score more than or equal to 12 points was considered an active case. The control sera were obtained from 20 healthy volunteers matched for age and sex.
The blood samples were obtained from all subjects and the serum was isolated and stored as frozen at − 80 °C for later ELISA experiments. The laboratory investigations were performed for all subjects. Serum complement levels (C3 and C4) were determined by using a quantitative competitive fluorescent probe technique, AssayLite™ Multiplex EFCIA Kit. Serum level of anti-phospholipid antibody (APL) (lupus anticoagulant) was measured by ELISA assay (MYBIOSOURCE kit). Serum levels of anticardiolipin antibody (ACL) were measured by using ELISA assay (DIAPHARMA kit); in addition, anti-double strand deoxyribonucleic acid (anti-dsDNA) and antinuclear antibody (ANA) concentrations were determined by using ELISA assay (SIGNOSIS kit). Serum levels of IL-17A, IFN-α and β were measured by using ELISA assay (ELABSCIENCE kit and RAYBiotech kit, respectively).
NFC measurements
NFC was performed for all subjects by a rheumatologist with a background in NFC using an XW 880 USB digital microscope with LED light, magnification power × 400, an 8 inch LCD monitor, and a 0.38 megapixel camera (Hefei Golden Brains optical instrument Co., China). Before the examination, each of the included subjects sat for 20 min in a room with a temperature of 20–24 °C. Both the middle and ring fingers from the right and left hands were examined to detect NFC abnormalities in Egyptian SLE patients. A drop of immersion oil was added to the nailfold bed to improve visualization. The following parameters were recorded: capillary density (number of capillaries counted in 1 mm area), capillary length (normal or elongated ≥ 300 μm), apical loop diameter including dilated capillaries (20–50 μm) and giant capillaries (> 50 μm), capillary morphology (shape of subject capillaries), and presence or absence of hemorrhages [16, 17]. According to Smith et al. [18], a qualitative analysis was adopted to evaluate capillaroscopic changes. The qualitative analysis includes three patterns: normal (pattern of typical hairpin-like capillaries with a regular distribution), non-specific (pattern with density, dimension, and morphological abnormalities but not meeting the definition of a ‘scleroderma pattern’), and scleroderma (pattern with giant capillaries, hemorrhages, and avascularity). Scleroderma pattern was divided into three subgroups (early, active, late) according to the degree of NFC abnormalities, where “early scleroderma pattern” was described as a pattern with normal density and capillary morphology, but has giant capillary(> 50 μm) with or without occurrence of hemorrhages, “active scleroderma pattern” was explained as a pattern with lowered density (4–6 capillary/mm), giant capillary (> 50 μm), abnormal morphology, and with presence or absence of hemorrhages, “late scleroderma pattern” was defined as a pattern with very low density (≤ 3 capillary/mm), abnormal morphology, and with absence of hemorrhages and giant capillaries. According to Sulli et al. [19], a semi quantitative analysis was also adopted which rates capillaroscopic changes from 0 to 3 (0 = no changes, 1 = ≤ 33% of capillary alterations/reduction, 2 = 33–66% of capillary alterations/reduction, 3 = ≥ 66% of capillary alterations/reduction, per linear mm).
Statistical analysis
The data were analyzed by t-test for unpaired samples and one way ANOVA to evaluate the significance of difference between means. The probability difference in frequency distributions was measured by using chi-square test or Fisher’s exact test. The correlation between data was performed by using Spearman’s rank coefficient. P value was considered significant when it was less than 0.05.
Results
The study contained 53 SLE patients with mean disease duration of 65 ± 30.6 months. SLEDAI ranged from 2 to 18 points, with a mean of 8.8 ± 3.7. 19 (35.85%) patients developed lupus nephritis. Arthritis was found in 14 (26.4%) patients and 8 (15.1%) patients showed malar rash. Five (9.4%), 21 (39.6%), and 2 (3.77%) SLE patients had alopecia, neurologic manifestations, and serositis, respectively. RP was observed in 8 patients (15.1%) of the 53 SLE patients. Leukopenia, defined as a total leukocyte count of less than 4000 cells/l, was found in 8 (15.1%) patients, and thrombocytopenia, defined as a low platelet count of less than 150,000 cells/l, was found in 9 (17%) patients. Fifteen patients (28.3%) tested positive for APL antibody. ACL antibody was discovered in 10 (18.9%) of the patients. There were 34 (64.15%) patients who took azathioprine drug, 21 (39.6%) patients who took hydroxychloroquine drug, and 51 (96.2%) patients who took prednisone drug. Table 1 presents the features of SLE patients.
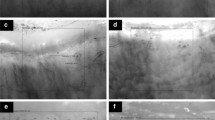
NFC changes were observed in 39 of 53 (73.6%) SLE patients, with 32 (60.4%) showing non-specific changes (Fig. 1) and 7 (13.2%) showing scleroderma pattern, including active scleroderma pattern in 3 (5.6%) (Fig. 2a), and late scleroderma pattern in 4 (7.55%) (Fig. 2b). Controls were compared to SLE patients in terms of age, sex, serum cytokine levels, and NFC changes as shown in Table 2. Serum levels of IL 17A, IFN α, and β were significantly higher (P < 0.0001) in SLE patients than in the control group. According to Table 2, most of SLE patients had elongated, crossed, tortuous, and dilated capillaries. In the control group, NFC changes were normal, with hairpin shape capillaries. When the previous two groups were compared in terms of NFC changes, there was a significant difference in capillary density (P = 0.02), capillary length (P = 0.0073), capillary elongation (P < 0.0001), capillary dilation (P = 0.015), and capillary tortuosity (P < 0.0001).
As shown in Table 3, NFC score has significant positive correlation with SLEDAI (P = 0.021). SLEDAI, on the other hand, showed significant positive correlations with disease duration, IL 17A, IFN α, and IFN β.
Table 4 shows the demographic and laboratory features of SLE patients based on NFC patterns (normal, non-specific, and scleroderma). RP was seen in three (21.43%) of SLE patients with a normal pattern and five (71.4%) of those with a scleroderma pattern. All patients with active scleroderma pattern (n = 3, 100%) and only two patients (50%) of four patients with late scleroderma pattern developed RP. Except for SLEDAI (P = 0.03) and the presence of RP (P < 0.0001), no significant differences in demographic and laboratory parameters of SLE patients were found between different capillaroscopic patterns. Patients with positive APL had different NFC patterns without any statistically significant difference.
Discussion
Many studies have recently focused on microvascular endothelial damage and its role in the pathogenesis of systemic organ involvement in rheumatic diseases like SLE [20]. Cytokines have a critical role in the development of SLE because they are important regulators of SLE pathogenesis and are linked to immune dysfunction and organ damage. The present study investigated the correlations between demographic, laboratory parameters, including IL 17A, IFN α, and IFN β, and NFC changes in Egyptian SLE patients to determine whether these parameters contribute to microvascular abnormalities, where the previous studies pointed to the great diagnostic value of NFC in rheumatic diseases, but there have been few studies on the association of immune system alterations with capillaroscopic abnormalities.
IL 17 cytokines are produced by many lymphocytes, including CD 4+ Th 17 cells, CD 8+ cells, γδ T cells, and natural killer T cells [21]. IL 17 family consists of six members (IL 17A to IL 17F) and the previous studies has found that IL 17A and IL 17F, in particular, can initiate tissue injury through the secretion of chemokines that cause monocytes recruitment, maturation, and proliferation. [22]. IL 17A is a cytokine with inflammatory properties that participates in the host defenses against bacterial and fungal infections as well as autoimmunity and tumors [23]. Studies reported that IL 17A is associated with SLE pathogenesis because it can activate B cells and cause local inflammation and tissue injury, which are associated with different events in the pathophysiology of SLE [24]. It can also magnify the immune response by increasing the secretion of autoantibodies by B lymphocyte activation, making it an appealing therapeutic target [25]. The present study demonstrated that individuals diagnosed with SLE and exhibiting active disease displayed elevated levels of IL-17A in comparison to those with mild or moderate disease and control subjects. Furthermore, a notable significant positive correlation was observed between IL 17A and SLEDAI. This correlation suggests that raised IL-17A levels contribute to increased disease activity or vice versa. These findings were consistent with previous studies' findings [26,27,28,29].
Type I IFNs, which are primarily secreted by plasmacytoid dendritic cells (pDCs), participate in the pathogenesis of SLE [30]. It was found that most of SLE symptoms are related to elevated levels of IFNs I. There are two types of cells that control vascular repair: bone marrow-derived EPCs and myelomonocytic circulating angiogenic cells (CACs) [31, 32]. Type I IFNs have a possibility of disrupt EPC/CAC function by decreasing pro-angiogenic factors such as VEGF and increasing IL-18 [32]. An in vitro study that found that blocking IFN-α in SLE patients' peripheral blood mononuclear cells (PBMCs) restored the normal angiogenic phenotype [31] provides evidence that type I IFNs promote abnormal vascular repair. The present study found elevated serum levels of type I IFNs in SLE patients when compared to the control group, with a significant positive correlation with SLEDAI, implying that as serum levels of IFNs I increase, disease activity increases, which is in agreement with the previous studies [33,34,35].
NFC has been used as a non-invasive method for detecting microvascular involvement in rheumatic diseases [11]. Capillary abnormalities have been reported in a variable prevalence in SLE patients [36,37,38]. Furthermore, the capillaroscopic changes in the nailfold appear to be related to the disease activity score [36]. Both the middle and ring fingers from the right and left hands were examined in the current study, as the previous multicenter studies showed that these fingers have a high sensitivity to detect capillary abnormalities [39, 40]. According to the findings of this study, 39 out of 53 (73.6%) SLE patients have NFC abnormalities, with non-specific pattern being the most prevalent pattern in SLE patients, followed by normal and scleroderma patterns; additionally, patients with scleroderma pattern showed only active and late pattern. The previous findings were consistent with other studies [41, 42] that found that the majority of SLE patients have a non-specific NFC pattern with rare occurrence of scleroderma pattern.
The tortuosity of capillaries exhibited a statistically significant increase in individuals diagnosed with SLE compared to the control group in this study. Tortuosity has been described as a normal variation in some studies [43, 44], but it has also been defined as an SLE pattern in others [11, 38]. Moreover, a notable significant difference in capillary density was obtained between the control group and the patient group. Specifically, a majority of individuals diagnosed with systemic lupus exhibited capillary density of below 7 capillary/mm. The prevailing NFC alterations observed in the Egyptian individuals with SLE include dilatation, elongation, and capillary crossing. These findings provide evidence to demonstrate that most SLE patients have non-specific abnormalities.
In addition, there was a correlation observed between changes in NFC and SLEDAI scores, whereas discernible variations in scleroderma patterns were observed between patients with inactive SLE and those with active SLE. Patients diagnosed with inactive SLE exhibited normal NFC, whereas active SLE patients had an abnormal pattern. These findings are consistent with many other studies that have found a correlation between capillary abnormalities and disease activity in SLE [45, 46]. Furthermore, the occurrence of hemorrhages was higher in active SLE cases than in inactive cases, which is consistent with the previous studies that demonstrated a higher frequency of hemorrhages in SLE patients with higher disease activity [47]. In contrast to the findings of Nakajima et al. [48], who found that NFC patterns correlated with subjects’ age, the current study found no significant correlation between NFC score and the ages of SLE patients.
The study found that SLE patients with different NFC patterns, including normal, nonspecific, and scleroderma, had no significant difference in IL 17A, IFN α, and β levels. Furthermore, there was no significant correlation between NFC score and previous serum cytokine levels, indicating that there was no clear relationship between NFC abnormalities and immunologic markers. When the previous three NFC patterns were compared based on the presence of RP, a significant difference was found, with scleroderma pattern patients having a higher occurrence of this phenomenon than the other two patterns, which was consistent with the findings of Furtado et al. [49], who discovered a significant correlation between the presence of RP and the scleroderma NFC pattern. Because no clinical signs of scleroderma were observed in the SLE patients with RP, these patients need close follow up for the possibility of the development of anti-U1 ribonucleoprotein antibodies (anti-U1 RNP) and mixed connective tissue diseases (MCTDs), as the previous studies [50, 51] discovered that the development of anti-U1 RNP antibodies is related to the presence of RP, which may lead to MCTDs in these patients. Moreover, no significant difference in antiphospholipid antibodies was found in the current study between different NFC patterns, which agrees with Raeeskarami et al. [52] who demonstrated that there was no significant correlation between antiphospholipid antibodies and capillary alterations and contrasts with other studies that used capillaroscopic changes as a diagnostic test for the antiphospholipid syndrome in rheumatic disease [42, 53, 54]. Our findings suggest that IL 17A and IFNs I have essential roles in the pathogenesis of SLE, but they may not have a specific effect on SLE microvascular abnormalities; additionally, NFC changes in Egyptian SLE patients are affected by disease activity rather than age or disease duration. NFC abnormalities in SLE can act as a mirror for microvascular involvement and disease activity.
Conclusion
The present study found that patients with SLE had elevated levels of IL 17A and IFNs I in their serum due to increased disease activity. Furthermore, the study's findings revealed that non-specific NFC patterns were more common in SLE patients. Notably, there was a significant positive correlation between NFC changes and disease activity but no correlation between NFC changes and studied cytokines. SLE patients with scleroderma NFC pattern and RP should be closely followed up for the possibility of appearance of anti-U1 RNP antibodies and MCTDS.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- SLE:
-
Systemic lupus erythematosus
- NFC:
-
Nailfold capillaroscopy
- CTDs:
-
Connective tissue diseases
- IL 17A:
-
Interleukin 17A
- IFN α:
-
Interferon alpha
- IFN β:
-
Interferon beta
- SLEDAI:
-
Systemic lupus erythematosus disease activity index
- VEGF:
-
Vascular endothelial growth factor
- pDCs:
-
Plasmacytoid dendritic cells
- EPCs:
-
Endothelial progenitor cells
- CACs:
-
Myelomonocytic circulating angiogenic cells
- PBMCs:
-
Peripheral blood mononuclear cells
- RP:
-
Raynaud’s phenomenon
- APL:
-
Anti-phospholipid antibody
- ACL:
-
Anticardiolipin antibody
- ESR:
-
Erythrocyte sedimentation rate
- Anti-U1 RNP:
-
Anti-U1 ribonucleoprotein antibodies
- MCTDs:
-
Mixed connective tissue diseases
References
Belmont HM, Abramson SB, Lie JT (1996) Pathology and pathogenesis of vascular injury in systemic lupus erythematosus. Interactions of inflammatory cells and activated endothelium. Arthritis Rheum 39:9–22
Hurrairah H, Ferro A (2004) The role of endothelium in the control of vascular function. Int J Clin Pract 58:173–183
Lee PY, Li Y, Richards HB, Chan FS, Zhuang H, Narain S et al (2007) Type I interferon as a novel risk factor for endothelial progenitor cell depletion and endothelial dysfunction in systemic lupus erythematosus. Arthritis Rheum 56:3759–3769
Yang J, Chu Y, Yang X, Gao D, Zhu L, Yang X et al (2009) Th17 and natural Treg cell population dynamics in systemic lupus erythematosus. Arthritis Rheum 60:1472–1483
Mok MY, Wu HJ, Lo Y, Lau CS (2010) The relation of interleukin 17 (IL-17) and IL-23 to Th1/Th2 cytokines and disease activity in systemic lupus erythematosus. J Rheumatol 37:2046–2052
Ronnblom LE, Alm GV, Oberg KE (1990) Possible induction of systemic lupus erythematosus by interferon-alpha treatment in a patient with a malignant carcinoid tumour. J Intern Med 227:207–210
Jiang J, Zhao M, Chang C, Wu H, Lu Q (2020) Type I interferons in the pathogenesis and treatment of autoimmune diseases. Clin Rev Allergy Immunol 59:248–272
Pan B, Shen J, Cao J, Zhou X, Shang L, Jin S et al (2015) Interleukin-17 promotes angiogenesis by stimulating VEGF production of cancer cells via the STAT3/GIV signaling pathway in non-small-cell lung cancer. Sci Rep 5:16053
Ferrara N (1999) Molecular and biological properties of vascular endothelial growth factor. J Mol Med 77:527–543
Lindner DJ (2002) Interferons as antiangiogenic agents. Curr Oncol Rep 4:510–514
Lambova SN, Muller-Ladner U (2013) Capillaroscopic pattern in systemic lupus erythematosus and undifferentiated connective tissue disease: what we still have to learn? Rheumatol Int 33:689–695
Cutolo M, Sulli A, Secchi ME, Pizzorni C (2006) Kapillarmikroskopie und rheumatische Erkrankungen: state of the art. Z Rheumatol 65:290–296
Ingegnoli F, Zeni S, Meani L, Soldi A, Lurati A, Fantini F (2005) Evaluation of nailfold videocapillaroscopic abnormalities in patients with systemic lupus erythematosus. J Clin Rheumatol 11:295–298
Tan EM, Cohen AS, Fries JF, Masi AT, Mcshane DJ, Rothfield NF et al (1982) The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 25:1271–1277
Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH (1992) Commitee on Prognosis Studies in SLE. Derivation of the SLEDAI. A disease activity index for lupus patients. Arthritis Rheum 35:630–640
Manfredi A, Sebastiani M, Cassone G, Pipitone N, Giuggioli D, Colaci M et al (2015) Nailfold capillaroscopic changes in dermatomyositis and polymyositis. Clin Rheumatol 34:279–284
Cutolo M, Sulli A, Smith V (2013) How to perform and interpret capillaroscopy. Best Pract Res Clin Rheumatol 27:237–248
Smith V, Vanhaecke A, Herrick AL, Distler O, Guerra MG, Denton CP et al (2019) Fast track algorithm: How to differentiate a “scleroderma pattern” from a “non-scleroderma pattern.” Autoimmun Rev 2019:102394
Sulli A, Secchi ME, Pizzorni C, Cutolo M (2008) Scoring the nailfold microvascular changes during the capillaroscopic analysis in systemic sclerosis patients. Ann Rheum Dis 67:885–887
Clancy R, Marder G, Martin V, Belmont HM, Abramson SB, Buyon J (2001) Circulating activated endothelial cells in systemic lupus erythematosus. Further evidence for diffuse vasculopathy. Arthritis Rheum 44:1203–1208
Dent AL, Kaplan MH (2008) T cell regulation of hematopoiesis. Front Biosci 13:6229–6236
Merayo-Chalico J, Barrera-Vargas A, Juárez-Vega G, Alcocer-Varela J, Arauz A, Gómez-Martín D (2018) Differential serum cytokine profile in patients with systemic lupus erythematosus and posterior reversible encephalopathy syndrome. Clin Exp Immunol 192:165–170
Iwakura Y, Ishigame H, Saijo S, Nakae S (2011) Functional specialization of interleukin-17 family members. Immunity 34:149–162
Rafael-Vidal C, Pérez N, Altabás I, Garcia S, Pego-Reigosa JM (2020) Blocking il-17: a promising strategy in the treatment of systemic rheumatic diseases. Int J Mol Sci 21:7100
Humrich JY, Riemekasten G (2019) Low-dose interleukin-2 therapy in refractory systemic lupus erythematosus: an investigator-initiated, single-centre phase 1 and 2a clinical trial. Lancet Rheumatol 1:e44–e54
Chen XQ, Yu YC, Deng HH, Sun JZ, Dai Z, Wu YW et al (2010) Plasma IL-17A is increased in new-onset SLE patients and associated with disease activity. J Clin Immunol 30:221–225
Rojas M, Rodríguez Y, León KJ, Pacheco Y, Acosta-Ampudia Y, Monsalve DM et al (2018) Cytokines and inflammatory mediators in systemic lupus erythematosus. EMJ Rheumatol 5:83–92
Wang D, Huang S, Yuan X, Liang J, Xu R, Yao G et al (2015) The regulation of the Treg/Th17 balance by mesenchymal stem cells in human systemic lupus erythematosus. Cell Mol Immunol 14:423–431
Tang Y, Tao H, Gong Y, Chen F, Li C, Yang X (2019) Changes of serum IL-6 IL-17, and complements in systemic lupus erythematosus patients. J Interferon Cytokine Res 39:410–415
Crow MK, Olferiev M, Kirou KA (2015) Targeting of type I interferon in systemic autoimmune diseases. Transl Res 165:296–305
Denny MF, Thacker S, Mehta H, Somers EC, Dodick T, Barrat FJ et al (2007) Interferon-alpha promotes abnormal vasculogenesis in lupus: a potential pathway for premature atherosclerosis. Blood 110:2907–2915
Cates AM, Holden VI, Myers EM, Smith CK, Kaplan MJ, Khlenberg JM (2015) Interleukin 10 hampers endothelial cell differentiation and enhances the effects of interferon α on lupus endothelial cell progenitors. Rheumatology (Oxford) 54:1114–1123
Sozzani S, Bosisio D, Scarsi M, Tincani A (2010) Type I interferons in systemic autoimmunity. Autoimmunity 43:196–203
Ronnblom L, Leonard D (2019) Interferon pathway in SLE: one ̈ key to unlocking the mystery of the disease. Lupus Sci Med 6:e000270
Yao Y, Higgs BW, Morehouse C, Reyes M, Trigona W, Brohawn P et al (2009) Development of potential pharmacodynamic and diagnostic markers for anti-IFN-α monoclonal antibody trials in systemic lupus erythematosus. Hum Genomics Proteomics 2009:374312
Cutolo M, Melsens K, Wijnant S, Ingegnoli F, Thevissen K, Keyser FD et al (2018) Nailfold capillaroscopy in systemic lupus erythematosus: a systematic review and critical appraisal. Autoimmun Rev 17:344–352
Smith V, Beeckman S, Herrick AL, Decuman S, Deschepper E, Keyser FD et al (2016) An EULAR study group pilot study on reliability of simple capillaroscopic definitions to describe capillary morphology in rheumatic diseases. Rheumatology (Oxford) 55:883–890
Bărbulescu AL, Vreju AF, Bugă AM, Sandu RE, Criveanu C, Tudoraşcu DR et al (2015) Vascular endothelial growth factor in systemic lupus erythematosus - correlations with disease activity and nailfold capillaroscopy changes. Rom J Morphol Embryol 56:1011–1016
Cutolo M, Herrick AL, Distler O, Becker MO, Beltran E, Carpentier P (2016) Nailfold videocapillaroscopic features and other clinical risk factors for digital ulcers in systemic sclerosis: a Multicenter, prospective cohort study. Arthritis Rheum 68:2527–2539
Dinsdale G, Roberts C, Moore T, Manning J, Berks M, Allen J et al (2018) Nailfold capillaroscopy—how many fingers should be examined to detect abnormality? Rheumatology 58:284–288
Higuera V, Amezcua-Guerra LM, Montoya H, Massó F, Patlán M, Paez A et al (2016) Association of Nail Dystrophy With Accrued Damage and Capillaroscopic Abnormalities in Systemic Lupus Erythematosus. J Clin Rheumatol 22:13–18
Shenavandeh S, Habibi S (2017) Nailfold capillaroscopic changes in patients with systemic lupus erythematosus: correlations with disease activity, skin manifestation and nephritis. Lupus 26:959–966
Ingegnoli F, Herrick AL (2013) Nailfold capillaroscopy in pediatrics. Arthritis Care Res 65:1393–1400
Riccieri V, Spadaro A, Ceccarelli F, Scrivo R, Germano V, Valesini G (2005) Nailfold capillaroscopy changes in systemic lupus erythematosus: correlations with disease activity and autoantibody profile. Lupus 14:521–525
Ingegnoli F (2013) Capillaroscopy abnormalities in relation to disease activity in juvenile systemic lupus erythematosus. Microvasc Res 87:92–94
Ciolkiewicz M, Kuryliszyn-Moskal A, Klimiuk PA (2010) Analysis of correlations between selected endothelial cell activation markers, disease activity, and nailfold capillaroscopy microvascular changes in systemic lupus erythematosus patients. Clin Rheumatol 29:175–180
Kuryliszyn-Moskal A, Klimiuk PA, Sierakowski S, Ciolkiewicz M (2007) Vascular endothelial growth factor in systemic lupus erythematosus: Relationship to disease activity, systemic organ manifestation, and nailfold capillaroscopic abnormalities. Arch Immunol Ther Exp (Warsz) 55:179–185
Nakajima T, Nakano S, Kikuchi A, Matsunaga Y (2022) Nailfold Capillary patterns correlate with age, gender, lifestyle habits, and fingertip temperature. PLoS ONE 17:e0269661
Furtado RN, Pucinelli ML, Cristo VV, Andrade LE, Sato EI (2002) Scleroderma-like nailfold capillaroscopic abnormalities are associated with anti-U1-RNP antibodies and Raynaud’s phenomenon in SLE patients. Lupus 11:35–41
Hoffman IE, Peene I, Meheus L, Huizinga TW, Cebecauer L, Isenberg D et al (2004) Specific antinuclear antibodies are associated with clinical features in systemic lupus erythematosus. Ann Rheum Dis 63:1155–1158
Chebbi P, Goel R, Ramya J, Gowri M, Herrick A, Danda D (2022) Nailfold capillaroscopy changes associated with anti-RNP antibodies in systemic lupus erythematosus. Rheumatol Int 42:1355–1361
Raeeskarami SR, Namazi N, Assari R, Najafizadeh SR, Hassannejad Z, Ziaee V (2020) The comparison of nailfold capillaroscopy between juvenile systemic lupus erythematosus and healthy controls: correlation with laboratory and clinical parameters. Int J Vascular Med 2020:7631958
Pyrpasopoulou A, Triantafyllou A, Anyfanti P, Douma S, Aslanidis S (2011) Capillaroscopy as a screening test for clinical antiphospholipid syndrome. Eur J Intern Med 22:e158–e159
Aslanidis S, Pyrpasopoulou A, Doumas M, Triantafyllou A, Chatzimichailidou S, Zamboulis C (2011) Association of capillaroscopic microhaemorrhages with clinical and immunological antiphospholipid syndrome. Clin Exp Rheumatol 29:307–309
Acknowledgements
The authors thank all staff members and colleagues in the Rheumatology and Immunology Department, Faculty of Medicine, Cairo University Hospitals, Egypt, for their helpful cooperation and all the study participants for their patience and support.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Mary Wadie and Mohamed Nasser. The first draft of the manuscript was written by Mohamed Nasser and revised by Azza El Amir and Alyaa Farid and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The current research has been conducted in accordance to the Declarations of Helsinki and after approval of the local Ethics Committee of Faculty of Medicine, Cairo University (Ethical approval code: D-1–2022). Written informed consents were obtained from all participants enrolled in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nasser, M., Wadie, M., Farid, A. et al. Nailfold capillaroscopy in Egyptian systemic lupus erythematosus (SLE) patients: correlation with demographic features and serum levels of IL 17A and IFNs I. Egypt Rheumatol Rehabil 50, 47 (2023). https://doi.org/10.1186/s43166-023-00215-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43166-023-00215-8