Abstract
Background
Rheumatoid arthritis (RA) is an autoimmune inflammatory disease. It is characterized by an inflammatory polyarthritis that preferentially affects the small joints leading to joint damage and eventual deformity and disability, and can also present with extra-articular manifestations. Micro RNA (miRNA) is a class of non-coding RNAs which negatively regulate messenger RNA (mRNA) expression. Several studies had shown that miRNA-23b has a close relationship with inflammation and autoimmune diseases. An increasing evidence has suggested that miRNA-23b is closely associated with many inflammatory and autoimmune diseases. The current study aimed to evaluate the plasma expression of miRNA-23b in rheumatoid arthritis (RA) patients and to explore its potential association with diseases activity.
Results
RA patients had a significantly higher plasma miRNA-23b expression than controls (P < 0.001). The miRNA-23b plasma expression was significantly associated with the clinical and laboratory indices of RA activity as well as with the DAS28-ESR score (P = 0.009) and grades (P < 0.001). The miRNA-23b plasma expression was significantly correlated with the radiological severity of RA (P = 0.002).
Conclusions
Plasma expression of miRNA-23b is significantly increased in patients with RA than controls. In RA patients, plasma expression of miRNA-23b was significantly correlated with the activity and radiological severity of RA. miRNA-23b may represent a potential therapeutic target that can retard progression of RA.
Similar content being viewed by others
Background
Rheumatoid arthritis (RA) is the most common autoimmune inflammatory arthritis. It affects approximately 0.5-1% of the population worldwide [1]. It is characterized by an inflammatory polyarthritis that preferentially affects the small joints leading to joint damage and eventual deformity and disability, and can also present with extra-articular manifestations, affecting other major organs in the body [2]. The etiopathogenesis of RA is not completely understood; however, it had been proposed that RA is a multifactorial disease and multiple genetic and environmental factors may contribute to the development RA [3].
In the past few years, there is increasing evidence pointing out that serum micro-ribonucleic acids (miRNAs) levels are elevated in patients with RA and are potentially associated with the pathogenesis of RA [4]. The miRNAs are a class of short, endogenous, non-coding RNAs of approximately 17–25 nucleotides [5], which negatively regulate messenger RNA (mRNA) expression. It leads to cleavage of target mRNA with subsequent degradation or translation inhibition of mRNA [6] resulting in disruption of crucial cellular processes, including cell growth, differentiation, and proliferation due to inhibition of protein synthesis [7]. Dysregulated miRNAs expression had been associated with many inflammatory and autoimmune conditions [8].
miRNA-23b belongs to the miRNA-23b/27b/3074/24-1 cluster, located in chromosome 9 [9]. Several studies had shown that miRNA-23b has a close relationship with inflammation and autoimmune diseases [10]. Overexpression of the miRNA-23b imposes a gene silencing effects on the recipient macrophages [11], upregulates the expression of proinflammatory cytokines in the vascular endothelial cells [12], and leads to suppression of dendritic cells maturation and differentiation [13]. More importantly, miRNA-23b level was found to be diminished in the synovial tissues in patients with RA after suppressing interleukin (IL)-17-associated autoimmune inflammation [14]. However, data available regarding plasma miRNA-23b expression in RA patients and its relationship with diseases activity are scanty.
The aim of this study was to evaluate the plasma expression of miRNA-23b in RA patients and to explore its potential association with diseases activity.
Methods
Participants
In this cross-sectional study, 100 consecutive patients diagnosed with RA according to the 2010 American College of Rheumatology/European League Against Rheumatism classification criteria [15] were invited to participate in the study. The patients were recruited during the period from January 2019 to January 2020 from the Outpatient Clinic of the Rheumatology and Rehabilitation Department. In addition, the study enrolled 100 healthy volunteers in the control group matched for age and gender with the RA group.
Subjects with history of autoimmune diseases, cardiovascular diseases, hepatic diseases, renal diseases, malignancy, and any other chronic diseases were excluded from the study. None of the participants were current or ex-smoker. Prior to inclusion in the study, the study aim, procedures, and any related information were described to the enrolled participants. Written consents were obtained from all participants and the study was approved by The University Ethics Committee (IRB/R.20.01.719) and in accordance with the Declaration of Helsinki.
Clinical assessment
All subjects underwent thorough medical history taking and thorough physical examination. Medical records of patients were reviewed. Data collected included the personal data (age and gender) for all participants. The medical history and findings including the disease duration, duration of morning stiffness, and drug intake were obtained from all RA patients. Clinical assessment of the RA patients included recording of the tender and swollen joint count (TJC and SJC respectively). Pain was assessed by the visual analog scale (VAS-pain). The disease activity was measured by the Disease Activity Score 28 (DAS28) based on erythrocyte sedimentation rate (ESR) level [16]. Patients with RA were classified according to DAS28-ESR as follows: remission (< 2.6); low disease activity (≥ 2.6 and ≤ 3.2); moderate disease activity (> 3.2 and ≤ 5.1); and high disease activity (> 5.1) [17].
Laboratory assessment
Adequate venous blood sample was collected under complete aseptic condition from every participant, between 9 and 10 a.m. after an overnight fasting in the same day of history taking and clinical evaluation. Samples were collected into two vacutainers: EDTA containing and serum separator. Serum was separated and used for autoantibodies detection including anticyclic citrullinated antibodies (anti-CCP) (Life Span BioScience, Inc.) by the automated immunoassay analyzer Immunolite 2000 (DPC Ltd., Gwynedd, UK) and Rheumatoid factor (RF) (Avitex RF). Part of EDTA anticoagulated whole blood was used for determination of ESR and C-reactive protein (CRP). The other part was separated as plasma for miRNA-23b study.
Measurement of plasma miRNA-23b expression
RNA extraction
Total RNA from plasma samples was extracted using Ribopure Blood RNA isolation kit according to the instructions of the manufacturer (Thermo Fisher Scientific Inc.). Isolated RNA quality and concentration were assessed on a NanoDrop ND 1000 spectrophotometer (NanoDrop Technologies, Wilmington, Delaware USA). Extracted samples of RNA were stored until use at −80 °C.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
TaqMan reverse transcription kit (Applied Biosystems; Thermo Fisher Scientific Inc.) was used for reverse transcription of miRNA-23b. The RT-qPCR was accomplished by the use of SYBR Green PCR Master Mix on an ABI 7300HT PCR system (Applied Biosystems; Thermo Fisher Scientific Inc.). The reaction was accomplished in a total volume of 20.0 μl containing 2.0 μl template (200 ng), 10.0 μl 2X SYBR Green Mix, 0.6 μl 200 nM forward and reverse primers, and 6.0 μl nuclease-free water. The used primers for miRNA-23b were forward, 5′-GAGCATCACATTGCCAGGG-3′ and reverse, 5′-GTGCAGGGTCCGAGGT-3′, while for U6 were forward, 5′-CTCGCTTCGGCAGCACATAT- 3′ and reverse, 5′-TTGCGTGTCATCCTTGCG-3′ (Invitrogen; Thermo Fisher Scientific Inc.). PCR conditions were as follows: Initial denaturation at 95 °C for 5 min, followed by 45 cycles at 95 °C for 30 s, 56 °C for 20 s, and 72 °C for 30 s. The relative expression level of miRNA-23b was normalized to the internal control RNU6B (U6) and were calculated by the 2–ΔΔCq normalization method (ΔCq = CqmiRNA-23b − CqU6) and reported as delta cycle threshold (deltaCt) [18].
Radiological assessment
Plain postero-anterior radiographs of hands, wrists, and feet bilaterally were obtained for all patients. Radiographs were evaluated based on the modified Sharp scoring method that comprised the determination of number of erosions and measurement of joint space narrowing [19].
All radiographs were scored by the same radiologist, who was blinded to the clinical and radiological data of the patients. All radiographs were interpreted twice with a 3-month interval by same radiologist. The inter-rater agreement for the radiographic score was 0.856.
Statistical analysis
IBM-SPSS software version 26.0 was used for statistical analysis. Continuous variables were tested for normality of distribution prior to statistical analysis. Plasma expression of miRNA-23b showed abnormal distribution and was expressed by median and interquartile range (IQR). The comparison of the plasma expression of miRNA-23b between RA patients group and controls group was performed using the Mann-Whitney U test. The other variables with continuous data showed normal distribution and were expressed as mean ± standard deviation (SD). Variables with categorical data were expressed as number and percentage. The correlation between the plasma expression of miRNA-23b and the variables containing continuous data was performed using the Pearson correlation test. The comparison of the miRNA-23b plasma expression among the DSA28-ESR activity grades was performed using the independent samples Kruskal-Wallis test. For all statistical procedures, significance threshold was set if P ≤ 0.05.
Results
Table 1 compares age and gender distribution between the RA patients group and controls group and the characteristics of the RA patients. Mean age and gender distribution did not show significant difference between the RA patients group and controls group.
Comparison of miRNA-23b plasma expression between RA patients group and controls group
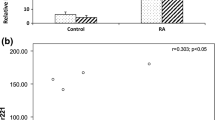
The median [IQR] of plasma expression of the miRNA-23b of the RA patients group was significantly higher than the controls (354.0 [557.0] and 34.0 [34.5] respectively, P < 0.001) (Fig. 1).
Correlation of miRNA-23b plasma expression with RA activity indices and radiological severity
As shown in Table 2, the plasma expression of the miRNA-23b of the RA patients group showed significant correlation with clinical activity indices: morning stiffness duration (P = 0.028), VAS-pain score (P = 0.011), TJC (P = 0.032), and SJC (P = 0.015). The plasma expression of the miRNA-23b of the RA patients group also showed significant correlation with CRP and ESR (P = 0.040 and P = 0.013 respectively). Similarly, the plasma expression of the miRNA-23b of the RA patients group showed significant correlation with the DAS28-ESR (P = 0.009). In addition, the plasma expression of the miRNA-23b of the RA patients group showed significant correlation with the modified Sharp score (P = 0.002).
On the other hand, miRNA-23b plasma expression did not show significant correlation with age, duration of RA, RF titer, and anti-CCP titer.
Comparison of miRNA-23b plasma expression among the DSA28-ESR activity grades
The median plasma expression of miRNA-23b (deltaCt) in the patients with remission was 178.0 [IQR = 400.0], with low disease activity was 225.0 [IQR = 200.0], with moderate disease activity was 374.0 [IQR = 555.0] and with high disease activity was 870.0 [IQR = 650.0]. These differences of the median plasma expression of miRNA-23b among the different DSA28-ESR activity grades were significant (P < 0.001) (Fig. 2).
Association of miRNA-23b plasma expression with gender and drug intake
The plasma miRNA-23b expression did not differ significantly between males and females. Similarly, plasma miRNA-23b expression was not significantly different between patients on and off medications (Table 3).
Discussion
miRNA-23b belongs to the miRNA-23b/27b/3074/24-1 cluster [9]. Several studies had shown that miRNA-23b has a close relationship with inflammation and autoimmune diseases [10].
The major findings of the present study were (a) RA patients had a significantly higher plasma miRNA-23b expression than controls; (b) the miRNA-23b plasma expression was significantly associated with the clinical and laboratory indices of activity as well as with the DAS28-ESR score and grades; and (c) the miRNA-23b plasma expression was significantly correlated with the radiological severity of RA.
Liu et al. enrolled 109 RA patients and 48 healthy controls. The study evaluated plasma and synovial tissues miRNA-23b expression and explored the value of miRNA-23b as a potential marker for RA activity. Results of that study revealed that RA patients show higher plasma of miRNA-23b than controls (median of 55.56 and 37.78 respectively) and the plasma level of miRNA-23b significantly correlated with the clinical and serological indicators of RA activity. The same study also reported that the synovial tissues of RA patients had significantly higher miRNA-23b than the matched controls [9]. These findings were consistent with our findings. Further, Liu et al. found that miRNA-23b plasma level in RA patients was reduced significantly after efficient treatment and parallel to the clinical improvement. Based on this finding, the study concluded that miRNA-23b is a promising marker that can indicate the degree of RA activity and therapeutic effectiveness in RA patients.
In contrast, the study by Andonian et al. [20] investigated relationships between six RA-related plasma miRNAs, including the miRNA-23b, and RA activity and proinflammatory cytokines was investigated and found that miRNA-23b, as well as all other miRNAs, showed no significant difference between the RA patients and controls. In addition, that study found no significant correlation between the plasma miRNAs with disease activity. These results were inconsistent to ours, and this discrepancy may be attributed to different methodological settings, smaller sample size, and genetic background. Indeed, the study of Andonian et al. included 48 RA patients and 23 controls and about 25% of the samples were African American while all participants in the present study were of Caucasian ethnicity.
Interestingly, a previous study investigated the role of miRNAs in inflammatory lesions-resident cells. The study observed a significantly lower miRNA-23b in the synovial tissues obtained from the patients with RA in comparison to the controls [14]. The same study observed that miRNA-23b is downregulated in lupus and multiple sclerosis inflammatory lesions. The study also found that IL-17 suppresses miRNA-23b expression in the fibroblast-like synovial cells. Of note, the number of synovial tissue samples obtained from controls and the patients in that study were 3 and 17, respectively. In the present study, the synovial fluid miRNA-23b was not investigated.
The association of the miRNA-23b with the inflammatory process in RA seems logical. In support to this hypothesis, an elevated miRNA-23b plasma expression promoted the T regulatory cells (Tregs) differentiation, while RA patients had decreased number of Tregs [10]. It is postulated that the plasma miRNA-23b upregulation is a result of the dramatic reduction of the number of Tregs in RA [21].
RA is a potentially destructive disease and can lead to considerable joint damage with intense impact on patient function and quality of life. In the current study, we explored the relationship between the serum miRNA-23b expression and the radiological severity in RA patients. Our results revealed a significant correlation between the serum miRNA-23b expression and the modified Sharp score. Tumor necrosis factor (TNF)-α is a potent inhibitory factor of osteogenic differentiation and activity of bone marrow mesenchymal stem cell (BMSC) [22]. TNF-α markedly induces miRNA-23b expression [2, 23]. Overexpression of miRNA-23b is a strong inhibitor to runx2, a main transcription factor in the process of osteogenesis, indicating that miRNA-23b may act as an endogenous factor that attenuates runx2 in the BMSCs [24]. Gathering these data together suggests that miRNA-23b is implicated in TNF-α-mediated reduction of BMSC osteogenesis via targeting runx2. These findings provide new insights into the regulatory role of miRNA-23b in BMSC osteogenic differentiation in inflammatory disease and development of bone erosions.
Finally, plasma miRNA-23b expression was not significantly different between patients on and off medication. This can be attributed to the lack of follow-up of patients in this cross-sectional study.
This present study had its limitations. The miRNA-23b expression in the synovial tissues or fluid was not investigated. In addition, it is a one center cross sectional study with no follow-up of patients with different medications. So, we recommend future studies with larger sample size and in multiple centers to be designed to address the contribution of miRNA in the inflammatory process of RA. Furthermore, follow-up studies are needed to explore the effect of medications on plasma miRNA-23b expression in RA patients. Also, studies that include other miRNAs and immunity modulators could get better insight on its diagnostic role and its value as a therapeutic target. Another area for future study is to explore whether targeting miRNA-23b can halt or diminish progression of RA.
Conclusions
Plasma expression of miRNA-23b is significantly increased in patients with RA than controls. In RA patients, plasma expression of miRNA-23b was significantly correlated with the activity and radiological severity of RA. miRNA-23b may represent a potential therapeutic target that can retard progression of RA.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- Anti-CCP:
-
Anti-cyclic citrullinated peptide
- BMSC:
-
Bone marrow mesenchymal stem cell
- CRP:
-
C-reactive protein
- DAS:
-
Disease activity score
- deltaCt:
-
Delta cycle threshold
- ESR:
-
Erythrocyte sedimentation rate
- IL:
-
Interleukin
- IQR:
-
Interquartile range
- miRNAs:
-
microRNAs
- mRNA:
-
messenger RNA
- RA:
-
Rheumatoid arthritis
- RF:
-
Rheumatoid factor
- SD:
-
Standard deviation
- SJC:
-
Swollen joint count
- TJC:
-
Tender joint count
- TNF:
-
Tumor necrosis factor
- Tregs:
-
T regulatory cells
- VAS:
-
Visual analog scale
References
McInnes IB, O’Dell JR (2010) State-of-the-art: rheumatoid arthritis. Ann Rheum Dis 69(11):1898–1906. https://doi.org/10.1136/ard.2010.134684
Guo Q, Wang Y, Xu D, Nossent J, Pavlos NJ, Xu J (2018) Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res 6(1):15. https://doi.org/10.1038/s41413-018-0016-9
Klareskog L, Padyukov L, Rönnelid J, Alfredsson L (2006) Genes, environment and immunity in the development of rheumatoid arthritis. Curr Opin Immunol 18(6):650–655. https://doi.org/10.1016/j.coi.2006.06.004
Churov AV, Oleinik EK, Knip M (2015) MicroRNAs in rheumatoid arthritis: altered expression and diagnostic potential. Autoimmun Rev 14(11):1029–1037. https://doi.org/10.1016/j.autrev.2015.07.005
Wang J, Chen J, Sen S (2016) MicroRNA as biomarkers and diagnostics. J Cell Physiol 231(1):25–30. https://doi.org/10.1002/jcp.25056
Condrat CE, Thompson DC, Barbu MG, Bugnar OL, Boboc A, Cretoiu D, Suciu N, Cretoiu SM, Voinea SC (2020) miRNAs as biomarkers in disease: latest findings regarding their role in diagnosis and prognosis. Cells 9(2):276
Hruskova V, Jandova R, Vernerova L, Mann H, Pecha O, Prajzlerova K, Pavelka K, Vencovsky J, Filkova M, Senolt L (2016) MicroRNA-125b: association with disease activity and the treatment response of patients with early rheumatoid arthritis. Arthritis Res Ther 18(1):124. https://doi.org/10.1186/s13075-016-1023-0
Alsaleh G, Suffert G, Semaan N, Juncker T, Frenzel L, Gottenberg JE, Sibilia J, Pfeffer S, Wachsmann D (2009) Bruton’s tyrosine kinase is involved in miR-346-related regulation of IL-18 release by lipopolysaccharide-activated rheumatoid fibroblast-like synoviocytes. J Immunol 182(8):5088–5097. https://doi.org/10.4049/jimmunol.0801613
Liu X, Ni S, Li C, Xu N, Chen W, Wu M, Wijnen AJ, Yuji Wang Y (2019) Circulating microRNA-23b as a new biomarker for rheumatoid arthritis. Gene 712:143911. https://doi.org/10.1016/j.gene.2019.06.001
Zheng J, Jiang HY, Li J, Tang HC, Zhang XM, Wang XR, Du JT, Li HB, Xu G (2012) MicroRNA-23b promotes tolerogenic properties of dendritic cells in vitro through inhibiting Notch1/NF-κB signalling pathways. Allergy 67(3):362–370. https://doi.org/10.1111/j.1398-9995.2011.02776.x
Ayyadurai S, Charania MA, Xiao B, Viennois E, Zhang Y, Merlin D (2014) Colonic miRNA expression/secretion, regulated by intestinal epithelial PepT1, plays an important role in cell-to-cell communication during colitis. PLoS One 9(2):e87614. https://doi.org/10.1371/journal.pone.0087614
Wu M, Gu JT, Yi B, Tang ZZ, Tao GC (2015) microRNA-23b regulates the expression of inflammatory factors in vascular endothelial cells during sepsis. Exp Ther Med 9(4):1125–1132. https://doi.org/10.3892/etm.2015.2224
Wu J, Ji C, Cao F, Lui H, Xia B, Wang L (2017) Bone marrow mesenchymal stem cells inhibit dendritic cells differentiation and maturation by microRNA-23b. Biosci Rep. 37(2):BSR20160436. https://doi.org/10.1042/BSR20160436
Zhu S, Pan W, Song X, Liu Y, Shao X, Tang Y, Liang D, He D, Wang H, Liu W, Shi Y, Harley JB, Shen N, Qian Y (2012) The microRNA miR-23b suppresses IL-17-associated autoimmune inflammation by targeting TAB2, TAB3 and IKK-α. Nat Med 18(7):1077–1086. https://doi.org/10.1038/nm.2815
Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd et al (2010) Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 62(9):2569–2581. https://doi.org/10.1002/art.27584
Prevoo ML, van’t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL (1995) Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 38(1):44–48. https://doi.org/10.1002/art.1780380107
Aletaha D, Ward MM, Machold KP, Nell VP, Stamm T, Smolen JS (2005) Remission and active disease in rheumatoid arthritis: defining criteria for disease activity states. Arthritis Rheum 52(9):2625–2636. https://doi.org/10.1002/art.21235
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)). Method Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Sharp JT, Young DY, Bluhm GB, Brook A, Brower AC, Corbett M, Decker JL, Genant HK, Gofton JP, Goodman N, Larsen A, Lidsky MD, Pussila P, Weinstein AS, Weissman BN, Sharp JT, Young DY, Bluhm GB, Brook A, Brower AC, Corbett M, Decker JL, Genant HK, Gofton JP, Goodman N, Larsen A, Lidsky MD, Pussila P, Weinstein AS, Weissman BN (1985) How many joints in the hands and wrists should be included in a score of radiologic abnormalities used to assess rheumatoid arthritis? Arthritis Rheum 28(12):1326–1335. https://doi.org/10.1002/art.1780281203
Andonian BJ, Chou CH, Ilkayeva OR, Koves TR, Connelly MA, Kraus WE, Kraus VB, Huffman KM (2019) Plasma microRNAs in established rheumatoid arthritis relate to adiposity and altered plasma and skeletal muscle cytokine and metabolic profiles. Front Immunol 10:1475. https://doi.org/10.3389/fimmu.2019.01475
Al-Zifzaf DS, Bakry SAE, Mamdouh R, Shawarby LA, Ghaffar AYA, Amer HA, Abd Alim A, Sakr HM, Abdel Rahman R (2015) FoxP3+T regulatory cells in rheumatoid arthritis and the imbalance of the Treg/TH17 cytokine axis. Egypt Rheumatol 37(1):7–15. https://doi.org/10.1016/j.ejr.2014.06.004
Marupanthorn K, Tantrawatpan C, Tantikanlayaporn D, Kheolamai P, Manochantr S (2015) The effects of TNF-α on osteogenic differentiation of umbilical cord derived mesenchymal stem cells. J Med Assoc Thai 98(Suppl 3):S34–S40
Deng L, Hu G, Jin L, Wang C, Niu H (2018) Involvement of microRNA-23b in TNF-α-reduced BMSC osteogenic differentiation via targeting runx2. J Bone Miner Metab 36(6):648–660. https://doi.org/10.1007/s00774-017-0886-8
Iaquinta MR, Lanzillotti C, Mazziotta C, Bononi I, Frontini F, Mazzoni E, Oton-Gonzalez L, Rotondo JC, Torreggiani E, Tognon M, Martini F (2021) The role of microRNAs in the osteogenic and chondrogenic differentiation of mesenchymal stem cells and bone pathologies. Theranostics 11(13):6573–6591
Acknowledgements
Not applicable.
Funding
No funding
Author information
Authors and Affiliations
Contributions
Study conception and design, laboratory investigations, statistical analysis, drafting and revising the manuscript by HMA, HKM, ME, and HA. SA and OG were responsible for data acquisition and analysis, statistical analysis, drafting and revising the manuscript. All authors read, critically revised the manuscript for important intellectual contents, and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by Mansoura University Ethics Committee (IRB /R.20.01.719) and in accordance with the Declaration of Helsinki, and written consents were obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdeen, H.M., Gharbia, O.M., Bassiouni, S.A.R.A.K. et al. Micro RNA-23b as a potential biomarker in rheumatoid arthritis disease activity and severity: clinical, laboratory, and radiological cross-sectional study. Egypt Rheumatol Rehabil 48, 39 (2021). https://doi.org/10.1186/s43166-021-00090-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43166-021-00090-1