Abstract
Background
There are few epidemiological data to support rehabilitation programs for cerebral palsy (CP). Scarce international studies described the developmental anomalies (DAs) among children with CP. To our knowledge, the Arab countries did not publish data regarding this topic. This study aimed to describe the percentage of DAs among children with CP and detect the association between clinical subtypes and impairment severity in children with various DAs. We collected registry data of 679 children with cerebral palsy, between 2014 and 2019, from Armed Forces Hospitals, Taif, Kingdom of Saudi Arabia (KSA). We recorded demographic, perinatal, postnatal, developmental anomalies, subtypes, and impairment characteristics. We utilized the chi-square test to calculate the differences between groups.
Results
We reported significant differences between the children with and without anomalies regarding the percentages of consanguinity, preterm labor, low birth weight, and neonatal intensive care unit admission (P = 0.001, 0.002, 0.003, 0.005, respectively). Congenital dysplasia of the hip and hydrocephalus was the most frequent skeletal and nervous anomalies among children with DAs (19.1% and 12.8%, respectively). The spastic bilateral pattern was significantly higher among children with skeletal anomalies than the central nervous system/other groups (P < 0.001). The nervous anomalies group had higher frequencies of severe intellectual, motor, speech, and visual disabilities and a higher percentage of seizures than all other groups.
Conclusions
The frequency of children with anomalies in this study was comparable to previous studies. Children with CP and nervous system anomalies had more severe motor disabilities and associated impairments.
Similar content being viewed by others
Background
Cerebral palsy (CP) is a collective group of permanent, non-progressive, developmental disorders affecting body movement, balance, and posture. It remains a worldwide common cause of pediatric morbidity and disability despite the scientific advances in neonatal and maternal care over the last decades. Children with CP had motor dysfunction often accompanied by sensory, perceptive, cognitive, communicating, learning, and behavioral disorders. Congenital or acquired musculoskeletal (MSK) complications are usually present [1, 2].
To date, CP and other developmental disorders have received insufficient attention in developing countries. Epidemiological evidence of pediatric disabilities, especially CP, in KSA is scarce [3]. In a community-based study, the prevalence of CP in KSA was 2.3/1000 population [4]. Another Saudi study covered 99,788 live births in Riyadh Military Hospital has reported a CP incidence of 0.41% [5]. Along with other neurological disorders of children, healthcare providers have not given CP high priority amongst health problems. Inadequate perinatal and postnatal care in developing countries makes it sensible to believe that CP incidence is much higher than in developed countries [6, 7].
Several risk factors associated with CP include multiple pregnancies, intrauterine growth restriction, preterm labor, neonatal asphyxia, and developmental anomalies (DAs) [8]. DAs involved wide-ranging abnormalities of body structure or function present at birth. These anomalies were due to genetic, teratogenic, or nutritional factors, or a combination of several factors. Studies showed that DAs were more common in children with CP than in the general pediatric population [9, 10]. The DAs were mostly in the central nervous system (CNS) across all Gross Motor Function Classification System (GMFCS) levels, and the presence of DA itself correlated with severity of CP. The genetic evaluation showed that 5q21.1 locus deletion linked with some CNS anomalies such as corpus callosum hypoplasia and microcephaly [11]. Thus, parents must obtain proper genetic counseling [12, 13].
Researchers reported that many risk factors of DAs included diabetes mellitus (DM), thyroid dysfunction, and drug toxicity. Maternal DM significantly increased the risk of DAs, including the CNS (microcephaly and spina bifida) and the skeletal system (sacral agenesis and limb defects) [14]. Moreover, the prevalence of DAs and Down syndrome were significantly higher among patients with primary hypothyroidism than the general population. Japanese patients had a higher frequency of cardiovascular anomalies than American and Egyptian patients, and a female predominance was also observed [15]. Previous experiments reported several medications, such as anti-epileptics, anti-convulsants, and anti-hypertensive, implicated in neural tube defects, cardiovascular, gastrointestinal, and urinary tract DAs [16].
Aim of the study
In this study, we aimed to describe the percentage of DAs among children with CP and detect the association between clinical subtypes and impairment severity in children with various DAs.
Methods
Study design and setting
In this multicenter observational analysis, we extracted the data, between 2014 and 2019, from Military Hospitals in Taif City, KSA. These hospitals included Armed Forces Rehabilitation Center, Alhada Hospital for Armed Forces, Prince Mansour Hospital for Society, and Prince Sultan Hospital for Armed Forces. The Wipro software system contained the registered pediatric population with CP. We used these registry-based medical archives to differentiate children with CP from other recorded patients.
Patients
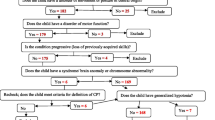
We reviewed 679 children with CP aged between 8 months and 17.8 years of both genders. We excluded the children with other neurological diagnoses or unconfirmed diagnosis of CP. Our definition of CP was the same as the definition used by European and Australian registries [17, 18]. Before registration, a multi-disciplinary team performed comprehensive evaluations to confirm the diagnosis and document the children’s disabilities. This team involved a physiatrist, audiologist, ophthalmologist, orthotist/prosthetist, dietician, psychologist, social counselor, physical, occupational, and speech therapists. After initial assessments, we subdivided the patients into children with CP and DAs (n = 188) and children with CP and without DAs (n = 491).
Study variables
The collected data included age at registration/years, registered year, gender (male/female), consanguinity (no/yes), mode of delivery (vaginal delivery/cesarean section), preterm defined as babies born alive < 37 weeks or 259 days gestation [19] (no/yes), low birth weight (LBW) defined as born weighing between 1500 and 2499 g [19] (no/yes), and admission to neonatal intensive care unit (NICU) (no/yes).
We classified DAs according to the World Health Organization (WHO)/the International Classification of Diseases version 11 (ICD-11) [19]. The skeletal anomalies included developmental dysplasia of the hip, congenital scoliosis, and pes planus. Reduction defects of the lower limb included femoral, tibial, or fibular hypoplasia (unilateral and bilateral). We reported the congenital deformities of the feet that involve talipes equinovarus, metatarsus varus, talipes calcaneovalgus, and hallux valgus. The nervous system anomalies included congenital hydrocephalus, microcephaly, corpus callosum agenesis, and spina bifida/ myelomeningocele). The European Surveillance of Congenital Anomalies (EUROCAT) defined microcephaly as a head circumference > 3 standard deviations (SD) below the mean [20]. We also documented the ocular (cataract), the respiratory (tracheomalacia), the circulatory (unspecified), the digestive, the genitourinary, and the chromosomal anomalies. According to the Surveillance of Cerebral Palsy in Europe (SCPE), we divided the clinical subtypes of CP into spastic unilateral or bilateral, dyskinetic, and ataxic [21].
The psychologist classified intellectual disability into mild (intelligence quotient [IQ] 50–69), moderate (IQ 36–49), severe (IQ 20–35), and profound (IQ < 20), according to the Diagnostic and Statistical Manual of Mental Disorders 5th edition (DSM-5) [22]. According to the GMFCS, we graded the motor impairment into mild (GMFCS levels I and II), moderate (GMFCS level III), and severe (GMFCS levels IV and V) (Appendix 1) [23, 24]. According to the American Speech-Language-Hearing Association, the speech therapist categorized speech disabilities into normal speech, mildly indistinct speech, moderately indistinct speech, severely indistinct speech, and mute (no speech) [25]. The ophthalmologist categorized visual acuity (VA) into normal, mild impairment (VA < 6/12), moderate impairment (VA < 6/18), severe impairment (VA < 6/60), and blind (VA < 3/60) using ICD-11 [19]. The audiologist defined the degree of hearing loss as normal (–10–15 decibel [dB]), minor (16–25 dB), mild (26–40 dB), moderate (41–55 dB), moderately severe (56–70 dB), severe (71–90 dB), and profound (> 91 dB) using pure-tone audiometry [26]. We documented seizures as present if they occurred at least once (absent/present).
Statistical analysis
We performed the statistical analysis using Statistical Package for Social Sciences (SPSS), version 23.0 (IBM SPSS®, Statistics 23, Armonk, NY, USA). We compared the data regarding demographic, perinatal, postnatal, clinical subtypes, and associated impairments. We applied the chi-square or Fisher’s exact tests to determine the differences between groups. We set the significant level at a probability (P) value < 0.05.
Results
We evaluated 679 children (386 males, 293 females) with CP. The mean age (SD) at registration was 7.8 (4.6) years. We reported significant differences between children with and without DAs regarding the prevalence of consanguinity (57.4% versus 13.2%, respectively), preterm labor (27.7% versus 17.0%, respectively), LBW (13.3 versus 6.3%, respectively), and admission to NICU (17.0% versus 9.4%, respectively). Age, gender, registered year, and mode of delivery showed insignificant differences between both groups (P > 0.05), as shown in Table 1. Congenital subluxation/dysplasia of the hip and hydrocephalus were the most frequent skeletal and nervous anomalies (19.1% and 12.8%, respectively). Twelve patients (6.4%) of the DAs group had Down syndrome, and 7.0% of all anomalies occurred in other systems (Table 2).
Children with skeletal DAs had higher unilateral spastic patterns than children without DAs and higher bilateral spasticity than children with nervous and other DAs. Meanwhile, no one of them had dyskinetic or ataxic subtypes. CP with nervous DAs had more severe intellectual, motor, speech, and visual disabilities than other groups (Table 3). Interestingly, patients with chromosomal anomalies (including Down syndrome) and nervous anomalies had near proportions of severe hearing impairment (9.7% and 9.0%, respectively). Seizures were significantly higher among the nervous anomalies’ group and significantly lower among the chromosomal/other anomalies group than the non-anomaly group (P = 0.003 and 0.02, respectively). The children with nervous anomalies had higher frequencies of intellectual, visual, and hearing impairments than the skeletal DAs group (P < 0.001, < 0.001, and 0.002, respectively). Motor and speech dysfunctions showed insignificant differences between skeletal and nervous systems groups, as demonstrated in Table 3.
Discussion
In the last decade, register-based studies have shown an increased interest in estimating DAs incidence among children with CP [27, 28]. However, no previous studies in KSA, to our knowledge, investigated the frequency of DAs in the CP population. So, we chose Taif city as a represented region of the Saudi people as it consisted of urban, rural, and sub-rural inhabitants. This study was the first Saudi, registry-based, multicenter research to describe DAs’ rate among CP children.
The prevalence of DAs among our CP children was 27.7%. The incidence of anomalies among children with CP varied substantially among different populations with a range between 11% and 40% [28,29,30,31,32,33,34,35,36]. The percentage of children with CP and DAs in our study (27.7%) was similar to Australian Cerebral Palsy Registry (28%) [18], higher than Jystad et al. (25%) [30], Rankin et al. (15%) [28], Pharoah (11%) [37], Garne et al. (12%) studies [17], and lower than described by Blair et al. (32%) [32]. Racial, ethnic, and sociocultural variations between studied populations explained the discrepancy in these studies. Besides, this inconsistency may be due to different inclusion criteria of the patients. Compared to our research, Blair et al. [32] only included minor DAs. Pharoah [37] and Garne et al. [17] depended on data from CP registries. Rankin et al. [28] utilized data from CP and DA registries.
The epidemiological figures of CP and DAs varied significantly worldwide, especially between low-, middle-, and high-income countries [38]. Considerable variations in the live birth rate of anomalies between nations were due to differences in prenatal screening and the proportion of pregnancy terminations [39, 40]. A higher proportion of pregnancy termination in women diagnosed with significant fetal DAs might be one reason clarifying the reported declines in CP and DAs rates [41, 42]. The interaction between genetic and environmental factors that differed among populations could also cause these variations [43].
In our studied population, the mean age at registration was about 8.0 years. This delayed registration for rehabilitation impaired the outcome of CP. The effectiveness of rehabilitative interventions of CP patients improved by early referral for physical therapy [44]. Maximal neural plasticity occurred during early childhood, where the therapeutic strategies had tremendous potential for long-term efficacy [45]. Moreover, early rehabilitation can prevent or modest the complex social, communication, and emotional associations that can have functional implications in later life [46]. We matched both groups (CP with and without DAs) regarding age at registration and gender (p > 0.05). So, these variables had an equal distribution among both groups, eliminating the impact of confounding variables on the study outcome [47]. Children with DAs had a higher consanguinity rate than children without DAs (13.3% versus 6.3%, respectively). The consanguinity rates in KSA and other Arab countries are significantly higher than in the Americans, Europeans, South-Africans, Eastern-Asians, and Oceanic countries [48]. The prevalence of consanguinity in the Saudi population is approximately 56% [49]. Consanguinity played an essential role in the etiopathogeneses of CP and DAs [10, 50,51,52]. Other significant differences between children with and without DAs included higher preterm labor, LBW, and NICU admission rates. Our findings confirmed the observations of earlier studies regarding CP/DAs risk factors [45, 53,54,55,56]. We found that the delivery mode was not significantly different between both groups, which was the same observation in the Turkish population [57].
In our study, most DAs presented in the skeletal system (53.7%), and about 30% appeared in the nervous system. This observation contrasted with prior studies where the CNS anomalies were the most common DAs [30, 36]. This result most likely reflected a referral pattern rather than a difference in epidemiology. Physicians referred CP children, to our rehabilitation center for physical therapy and functional rehabilitation, mainly due to MSK dysfunction. CP rehabilitation goals were primarily to minimize MSK complications rather than treating the central neurological deficit [44].
We can easily say that the most common DAs in our patients affecting the MSK and CNS. These findings are comparable to previous hospital-based studies [36, 58, 59]. Skeletal and nervous anomalies are widespread anomalies since they are physically apparent, and parents seek early medical advice [58, 59]. Out of 188 patients with DAs, only 5 (2.7%) had cardiovascular DAs, much lower than other studies (10-14%) [28, 30]. Survival bias explained these findings—this type of selection bias disproportionate the survivors of a particular outcome and ignored the non-survivors. Focusing on the survivors resulted in a false-negative estimate of probability [60]. Unfortunately, our results may underestimate the actual incidence of DAs among the CP population.
Our data revealed that congenital subluxation and dysplasia of the hip account for 19.1% of all anomalies in CP patients with DAs. Hydrocephalus was the most recorded DAs of the nervous system (12.8%). The muscle weakness affecting the limb positioning in utero increased the risk of congenital hip affection in CP children. Likewise, the detectable risk of hydrocephaly is the consequence of prenatal brain affection. The co-occurrence of these DAs with CP approved that cerebral dysfunction happens during fetal growth. The brain impairment led to neuronal migration abnormalities, encephalopathy, and other cerebral pathologies presented clinically as CP [37].
Following former CP studies, we found that spastic CP was the most common clinical subtype (93.1%) [5, 6, 28, 51]. Ataxic CP was the least frequent type, with a prevalence of 2.8%. This result was consistent with Rankin et al. [28] and Taha and Mahdi [51] observations but differed from Garne’s study, where the dyskinetic type was more frequent than other types [17]. Our systematic neurodevelopmental evaluation of each patient documented the motor impairment subtypes’ spastic, ataxic, or dyskinetic. The clinical features of motor affection were defined for each extremity differentiating unilateral from bilateral involvement (spastic unilateral or bilateral) [21]. The present study emphasized the results of Jystad and his colleagues [30] that children with skeletal DAs had a higher frequency of spastic CP than other children. Spasticity is a crucial factor in MSK complications of CP because spastic agonist muscles overcome the weaker antagonist muscles causing contractures and deformities. Hip flexors/adductors, hamstrings, gastrocnemius, soleus, and paraspinal muscles are most affected. Spasticity causes several bony and articular changes, including hip subluxation/ dislocation, foot deformities, and scoliosis [61].
We found that children with the DAs of CNS had more severe psychomotor, vision, hearing, speech impairments, and a higher frequency of seizures than other children. Jystad et al. [30] observed that children with CNS-anomalies had more severe motor impairments, impaired speech function, a higher proportion of epilepsy, and profound vision and hearing impairments than children without anomalies. These findings were also in line with Blair et al. [32], who reported a higher risk for severe CP and associated impairments in children with CNS anomalies. Comparably, Manlongat et al. [36] found that poor functional outcomes were more frequent in children with neurological anomalies than in children without neurological anomalies. Non-verbal communication, epilepsy, blindness, and bilateral disorders were observed exclusively in children with neurological DAs.
The WHO recommended implementing community genetics programs to prevent DAs at the population level. This program’s policies included neonatal screening for preventable diseases, detecting carriers of common recessive conditions, and removing environmental factors causing these disorders [62]. Successful programs in the Middle East and North/South Africa included Human Health in Africa, the Qatar Genome Program, and the Mexico National Institute of Genomic Medicine [63].
This study had several strengths. A confirmed diagnosis of CP was accomplished by the multi-disciplinary team before registration using a thorough neurodevelopmental assessment. Most of the findings were highly statistically significant (P < 0.001). These results indicated that the observations from data generated by significant tests were not likely to occur randomly or by chance but due to a specific cause [64]. In contrast to Manlongat et al. [36], we collected the data from registry-based medical archives, not from manual medical records. We kept the missing data to the minimum. We identified DAs by clinical examinations, X-rays, neuroimaging, ultrasonography, echocardiography, and genetic testing.
We found some limitations worth to be mentioned. The study did not include all CP children. Hospitals of the Ministry of Health had their cases, which might not refer to us. Additionally, we may not determine some children with mild CP [55]. Furthermore, the retrospective nature of the current study may be one of the potential limitations. However, we treated the disadvantage of retrospective design by matching children with and without DAs for age and gender, which enhanced the equal representation of subjects and reduced selection bias [65].
Conclusions
The proportion of children with DAs in Taif, KSA (27.7%) was comparable to that identified in many other countries. Children with CP and DAs, mainly nervous system DAs, had more severe motor disabilities and associated impairments. We proposed that DAs must be one of the primary information included in every newly developed CP registry in other regions. These data will accelerate early intervention to minimize the severity of associated disabilities and improve CP patients’ quality of life. Further research may provide successful opportunities for primary prevention.
Availability of data and materials
The authors confirm that the data supporting the findings of this study are available on request.
Abbreviations
- CNS:
-
Central nervous system
- CP:
-
Cerebral palsy
- DAs:
-
Developmental anomalies
- dB:
-
Decibel
- DSM-5:
-
Diagnostic and Statistical Manual of Mental Disorders 5th edition
- EUROCAT:
-
European Surveillance of Congenital Anomalies
- GMFCS:
-
Gross Motor Function Classification System
- ICD-11:
-
International Classification of Diseases version 11
- IQ:
-
Intelligence quotient
- KSA:
-
Kingdom of Saudi Arabia
- LBW:
-
Low birth weight
- MSK:
-
Musculoskeletal
- NICU:
-
Neonatal intensive care unit
- P:
-
Probability
- SD:
-
Standard deviation
- SCPE:
-
Surveillance of Cerebral Palsy in Europe (SCPE)
- SPSS:
-
Statistical Package for Social Sciences (SPSS)
- VA:
-
Visual acuity
- WHO:
-
World Health Organization
References
Panteliadis CP, Hagel C, Karch D, Heinemann K (2015) Cerebral palsy: a lifelong challenge asks for early intervention. Open Neurol J 9:45
Blair E, Cans C (2018) The definition of cerebral palsy. In: Cerebral palsy. Springer, pp 13–17
Shepherd RB (2014) Cerebral palsy in infancy. 1st Ed. Shepherd R, editor. Elsevier Health Sciences, Churchill Livingstone, p 29
Al Salloum AA, El Mouzan MI, Al Omar AA, Al Herbish AS, Qurashi MM (2011) The prevalence of neurological disorders in Saudi children: a community-based study. J Child Neurol 26(1):21–24
Al-Asmari A, Al Moutaery K, Akhdar F, Al JM (2006) Cerebral palsy: incidence and clinical features in Saudi Arabia. Disabil Rehabil 28(22):1373–1377
Al-Sulaiman AA, Bademosi OF, Ismail HM, Al-Quliti KW, Al-Shammary SF, Abumadini MS et al (2003) Cerebral palsy in Saudi children. Neurosciences. 8(1):26–29
Lagunju IA, Fatunde OJ (2009) The child with cerebral palsy in a developing country--diagnosis and beyond. J Pediatr Neurol 7(4):375–379
Stavsky M, Mor O, Mastrolia SA, Greenbaum S, Than NG, Erez O (2017) Cerebral palsy—trends in epidemiology and recent development in prenatal mechanisms of disease, treatment, and prevention. Front Pediatr 5:21
Goldsmith S, Jalon GG, Badawi N, Blair E, Garne E, Gibson C et al (2018) Comprehensive investigation of congenital anomalies in cerebral palsy: protocol for a European-Australian population-based data linkage study (The Comprehensive CA-CP Study). BMJ Open 8(7):e022190
Shawky RM, Sadik DI (2011) Congenital malformations prevalent among Egyptian children and associated risk factors. Egypt J Med Hum Genet 12(1)
Fahey MC, Maclennan AH, Kretzschmar D, Gecz J, Kruer MC (2017) The genetic basis of cerebral palsy. Dev Med Child Neurol 59(5):462–469
Williams J (2017) Congenital anomalies and cerebral palsy: cause or comorbidity? Dev Med Child Neurol 59(11):1108
Ashwal S, Russman BS, Blasco PA, Miller G, Sandler A, Shevell M et al (2004) Practice parameter: diagnostic assessment of the child with cerebral palsy: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 62(6):851–863
Chen C-P (2005) Congenital malformations associated with maternal diabetes. Taiwan J Obstet Gynecol 44(1):1–7
Gu Y-H, Harada S, Kato T, Inomata H, Aoki K, Hirahara F (2009) Increased incidence of extrathyroidal congenital malformations in Japanese patients with congenital hypothyroidism and their relationship with Down syndrome and other factors. Thyroid. 19(8):869–879
Offor I, Awodele O, Oshikoya KA (2019) Drug-related teratogenic and pathologic causes of birth defects in a tertiary hospital in Southwestern Nigeria. Pharmacol Res Perspect 7(1):e00452
Garne E, Dolk H, Krägeloh-Mann I, Ravn SH, Cans C (2008) The European Cerebral Palsy Database (SCPE) Collaborative Group. Cerebral palsy and congenital malformations. Eur J Paediatr Neurol 12(2):82–88
Australian Cerebral Palsy Register. Australian cerebral palsy register report. 2018;(December):18. Available from: https://cpregister.com/wp-content/uploads/2019/02/Report-of-the-Australian-Cerebral-Palsy-Register-Birth-Years-1995-2012.pdf
World Health Organization (WHO) (2020) International classification of diseases for mortality and morbidity statistics (11th Revision), 11th edn Available from: https://icd.who.int/browse11/l-m/en
European Surveillance of Congenital Anomalies (EUROCAT). European Surveillance of Congenital Anomalies (EUROCAT) Guide 1.4: instruction for the registration of congenital anomalies. EUROCAT Central Registry, University of Ulster. 2013;(Last update version 28/12/2018):1–161. Available from: https://eu-rd-platform.jrc.ec.europa.eu/sites/default/files/Full_Guide_1_4_version_28_DEC2018.pdf
Cans C (2000) Surveillance of cerebral palsy in Europe: a collaboration of cerebral palsy surveys and registers. Dev Med Child Neurol 42(12):816–824
American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders (DSM-5®), 5th edn. American Psychiatric Pub Available from: https://doi.org/10.1176/appi.books.9780890425596
Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B (1997) Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol 39(4):214–223
Reid SM, Carlin JB, Reddihough DS (2011) Using the Gross Motor Function Classification System to describe patterns of motor severity in cerebral palsy. Dev Med Child Neurol 53(11):1007–1012
American Speech-Language-Hearing Association (2004) Preferred practice patterns for the profession of speech-language pathology. ASHA Available from: https://www.asha.org/policy/pp2004-00191/
Clark JG (1981) others. Uses and abuses of hearing loss classification. ASHA. 23(7):493–500
McIntyre S, Blair E, Badawi N, Keogh J, Nelson KB (2013) Antecedents of cerebral palsy and perinatal death in term and late preterm singletons. Obstet Gynecol 122(4):869–877
Rankin J, Cans C, Garne E, Colver A, Dolk H, Uldall P et al (2010) Congenital anomalies in children with cerebral palsy: a population-based record linkage study. Dev Med Child Neurol 52(4):345–351
Australian Cerebral Palsy Register Group (2016) Report of the Australian Cerebral Palsy Register, Birth Years 1995-2014. Cereb Palsy Alliance Res Inst Sydney:48–53
Jystad KP, Strand KM, Bjellmo S, Lydersen S, Klungsöyr K, Stoknes M et al (2017) Congenital anomalies and the severity of impairments for cerebral palsy. Dev Med Child Neurol 59(11):1174–1180
Smithers-Sheedy H, McIntyre S, Gibson C, Meehan E, Scott H, Goldsmith S et al (2016) A special supplement: findings from the Australian Cerebral Palsy Register, birth years 1993 to 2006. Dev Med Child Neurol 58:5–10
Blair E, Al Asedy F, Badawi N, Bower C (2007) Is cerebral palsy associated with birth defects other than cerebral defects? Dev Med Child Neurol 49(4):252–258
Pharoah POD (2007) Prevalence and pathogenesis of congenital anomalies in cerebral palsy. Arch Dis Childhood-Fetal Neonatal Ed 92(6):F489–F493
Garne E, Dolk H, Krägeloh-Mann I, Ravn SH, Cans C, Group SC (2008) Cerebral palsy and congenital malformations. Eur J Paediatr Neurol 12(2):82–88
Croen LA, Grether JK, Curry CJ, Nelson KB (2001) Congenital abnormalities among children with cerebral palsy: more evidence for prenatal antecedents. J Pediatr 138(6):804–810
Manlongat E, Mcintyre S, Smithers-Sheedy H, Trivedi A, Muhit M, Badawi N et al (2020) Congenital anomalies in children with cerebral palsy in rural Bangladesh. Dev Med Child Neurol 62(4):463–469
Pharoah POD (2007) Prevalence and pathogenesis of congenital anomalies in cerebral palsy. Arch Dis Child Fetal Neonatal Ed 92(6):489–493
Khandaker G, Smithers-Sheedy H, Islam J, Alam M, Jung J, Novak I et al (2015) Bangladesh Cerebral Palsy Register (BCPR): a pilot study to develop a national cerebral palsy (CP) register with surveillance of children for CP. BMC Neurol 15(1):173
Bryant AG, Grimes DA, Garrett JM, Stuart GS (2011) Second-trimester abortion for fetal anomalies or fetal death: labor induction compared with dilation and evacuation. Obstet Gynecol 117(4):788–792
Kerns J, Vanjani R, Freedman L, Meckstroth K, Drey EA, Steinauer J (2012) Women’s decision making regarding choice of second trimester termination method for pregnancy complications. Int J Gynecol Obstet 116(3):244–248
Reid SM, Meehan E, McIntyre S, Goldsmith S, Badawi N, Reddihough DS et al (2016) Temporal trends in cerebral palsy by impairment severity and birth gestation. Dev Med Child Neurol 58:25–35
Sellier E, Platt MJ, Andersen GL, Krägeloh-Mann I, De La Cruz J, Cans C et al (2016) Decreasing prevalence in cerebral palsy: a multi-site European population-based study, 1980 to 2003. Dev Med Child Neurol 58(1):85–92
Moreno-De-Luca A, Ledbetter DH, Martin CL (2012) Genetic insights into the causes and classification of the cerebral palsies. Lancet Neurol 11(3):283–292
Içaugasiouglu A, Mesci E, Yumusakhuylu Y, Turgut ST, Murat S (2015) Rehabilitation outcomes in children with cerebral palsy during a 2 year period. J Phys Ther Sci 27(10):3211–3214
Maitre NL, Slaughter JC, Aschner JL (2013) Early prediction of cerebral palsy after neonatal intensive care using motor development trajectories in infancy. Early Hum Dev 89(10):781–786
Krakovsky G, Huth MM, Lin L, Levin RS (2007) Functional changes in children, adolescents, and young adults with cerebral palsy. Res Dev Disabil 28(4):331–340
De Graaf MA, Jager KJ, Zoccali C, Dekker FW (2011) Matching, an appealing method to avoid confounding? Nephron Clin Pract 118(4):c315–c318
El-Hazmi MA, Al-Swailem AR, Warsy AS, Al-Swailem AM, Sulaimani R, Al-Meshari AA (1995) Consanguinity among the Saudi Arabian population. J Med Genet 32(8):623–626
Middle I (2007) Regional variations in the prevalence of consanguinity in Saudi Arabia. Saudi Med J 28(12):1881–1884
Daher S, El-Khairy L (2014) Association of cerebral palsy with consanguineous parents and other risk factors in a Palestinian population. East Mediterr Health J 20(7):459–468
Taha SA, Mahdi AH (1984) Cerebral palsy in Saudi Arabia: a clinical study of 102 cases. Ann Trop Paediatr 4(3):155–158
Sinha G, Corry P, Subesinghe D, Wild J, Levene MI (1997) Prevalence and type of cerebral palsy in a British ethnic community: the role of consanguinity. Dev Med Child Neurol 39(4):259–262
Behrman RE, Butler AS et al (2007) Preterm birth: causes, consequences, and prevention, vol 772. National Academies Press, Washington, DC
Parker W, Hornik CD, Bilbo S, Holzknecht ZE, Gentry L, Rao R et al (2017) The role of oxidative stress, inflammation and acetaminophen exposure from birth to early childhood in the induction of autism. J Int Med Res 45(2):407–438
Khandaker G, Muhit M, Karim T, Smithers-Sheedy H, Novak I, Jones C et al (2019) Epidemiology of cerebral palsy in Bangladesh: a population-based surveillance study. Dev Med Child Neurol 61(5):601–609
Moore T, Hennessy EM, Myles J, Johnson SJ, Draper ES, Costeloe KL et al (2012) Neurological and developmental outcome in extremely preterm children born in England in 1995 and 2006: the EPICure studies. Bmj. 345:e7961
Öztürk A, Demirci F, Yavuz T, Y\ild\iz S, De\ugirmenci Y, Dö\cso\uglu M et al (2007) Antenatal and delivery risk factors and prevalence of cerebral palsy in Duzce (Turkey). Brain and Development 29(1):39–42
Fatemaq K, Begum F, Akter N, Zaman SMM (2011) Major congenital malformations among the newborns in BSMMU hospital. Bangladesh Med J 40(1):7–12
Shuma ML, Halder S, Datta BK (2015) Epidemiology of congenital anomalies among the children born in different hospitals under Sylhet Division in Bangladesh-a retrospective study. Dhaka Univ J Pharm Sci 14(2):225–230
Elton EJ, Gruber MJ, Blake CR (1996) Survivor bias and mutual fund performance. Rev Financ Stud 9(4):1097–1120
Morrell DS, Pearson JM, Sauser DD (2002) Progressive bone and joint abnormalities of the spine and lower extremities in cerebral palsy. Radiographics. 22(2):257–268
Kingsmore SF, Lantos JD, Dinwiddie DL, Miller NA, Soden SE, Farrow EG et al (2012) Next-generation community genetics for low-and middle-income countries. Genome Med 4(3):1–8
Tekola-Ayele F, Rotimi CN (2015) Translational genomics in low-and middle-income countries: opportunities and challenges. Public Health Genomics 18(4):242–247
Skelly AC (2011) Probability, proof, and clinical significance. Evid Based Spine Care J 2(4):9
Sedgwick P (2014) Retrospective cohort studies: advantages and disadvantages. BMJ Br Med J 348:g1072
Acknowledgements
We appreciate all the participants in this study. The contribution of our colleagues in the multi-disciplinary team is also invaluable.
Funding
The authors did not receive any financial support for the research, authorship, and publication of this study.
Author information
Authors and Affiliations
Contributions
SA, SD, and AIM initiated the study question, design, and protocol. SD and AIM performed the data collection. All authors agreed both to be personally accountable for the author’s contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. AIM completed the data management and statistical analysis, and HS revised the results and achieved the discussion with AIM. The authors have read the draft and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This research has been reviewed and accepted by the Directors and the Research Ethical Committee of Armed Forces Hospitals (Reg. No. H-02-T-078, Ref. REC.T. 2020-461). We prepared the study according to the principles expressed in the Helsinki Declaration. All parents signed/fingerprinted an informed written consent included the aim and the benefits of the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Al-Garni, S., Derbala, S., Saad, H. et al. Developmental anomalies and associated impairments in Saudi children with cerebral palsy: a registry-based, multicenter study. Egypt Rheumatol Rehabil 48, 9 (2021). https://doi.org/10.1186/s43166-021-00057-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43166-021-00057-2