Abstract
Background
Tympanic membrane perforations are among the most common indications for otological surgery. Surgeons from all around the world have made accidental and intentional contributions to find the perfect graft material and the best approach to perform tympanoplasty. Platelet-rich fibrin (PRF) use in myringoplasty has been popular in the last decade among many surgeons. We aimed to evaluate the efficacy of using PRF only as a graft material in myringoplasty.
Methods
We conducted a prospective study including 20 patients with dry central tympanic membrane perforations. The patients underwent endoscopic transcanal myringoplasty using a platelet-rich fibrin membrane by underlay technique. Follow-up was done 3 months postoperatively regarding graft uptake and hearing outcome by audiological assessment using pure tone audiometry.
Results
Sixty percent of patients were surgically successful with complete healing of the tympanic membrane, 10% were partially successful with partial healing of the tympanic membrane, and 30% failed with complete loss of graft. The mean air-bone gap closure was 3.5 dB. No major complications were reported.
Conclusion
Endoscopic myringoplasty using platelet-rich fibrin alone can be performed in cases with tympanic membrane perforations as an alternative to using other autologous grafts. It is a new reliable method for myringoplasty, as well as being an easy, time-saving procedure that avoids the need for any skin incision.
Similar content being viewed by others
Background
Myringoplasty is a surgical procedure to repair tympanic membrane perforations and is confined to the ear drum without manipulation of the ossicles or the middle ear [1].
There are two primary graft placement techniques: medial grafting (or underlay) and lateral grafting (or overlay). These terms refer to the position of the graft in relation to the fibrous annulus, the fibrous layer of the tympanic membrane remnant [2]. Underlay tympanoplasty is relatively quick and easy to perform. The advantages of the underlay technique also include the avoidance of anterior blunting and lateralization of the graft, which are the major disadvantages of the overlay technique, which is also a technically difficult technique [3]. Disadvantages include reduction of the middle ear space, formation of adhesions in the middle ear, and limited bed size for the graft in comparison with the overlay technique [3].
The two most commonly used grafting materials are temporalis fascia and cartilage [4]. Other graft materials were also used, including perichondrium, fat, and allografts such as gelfilm or fibrin glue [5,6,7].
Platelet-rich fibrin (PRF) is a second-generation platelet concentrate that is prepared without the use of anti-coagulants [8]. PRF is formed of many cells (mainly platelets and leukocytes) that are trapped within a fibrin matrix together with growth factors [9]. PRF fulfills all three essential requirements for tissue regeneration: it serves as a three-dimensional fibrin scaffold; it contains autologous cells like neutrophils, platelets, leukocytes, and macrophages; and it acts as a reservoir for natural growth factors that can be released over a 10- to 14-day period [10].
This study aimed to assess the effectiveness of using platelet-rich fibrin (PRF) in endoscopic myringoplasty with regard to the graft take rate and hearing outcome.
Methods
We conducted our study on 20 patients presented with safe dry central tympanic membrane perforations admitted to the Otorhinolaryngology Department, Faculty of Medicine, Cairo University. This study included all patients diagnosed with inactive mucosal chronic suppurative otitis media for at least 12 months. All our patients have conductive hearing loss with an air-bone gap ≤ 30 dB. Patients with cholesteatoma, attic tympanic membrane perforation, or suspected ossicular pathology (> 40 dB air-bone gap) were excluded. Also, we excluded patients with any medical co-morbidity that may affect graft take, such as anemia, diabetes mellitus, or any other chronic debilitating disease. All patients underwent full history-taking and general and otoscopic ear examinations. A preoperative complete blood count was ordered to determine the platelet count to assess the feasibility of PRF administration. Preoperative audiological assessment by pure tone audiometry was done.
Preparation of the platelet-rich fibrin
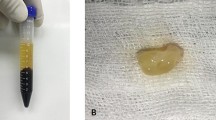
Before induction of general anesthesia, a 10-ml blood sample was taken from each patient using a 10-ml syringe with a 20-gauge needle. The blood was immediately evacuated into a sterile plain tube. The tube was centrifuged using a tabletop centrifuge at 2700 rounds per minute for 12 min [8]. Centrifugation resulted in the formation of a fibrin clot, a buffy jelly-like layer of platelet-rich fibrin in the middle of the tube (between the RBC layer at the bottom and platelet-poor plasma at the top) (Fig. 1). This middle layer was held outside the tube with a non-toothed forceps and put on a piece of gauze under complete aseptic precautions (Fig. 2). The PRF layer was kept to dry while performing the surgical procedure (Fig. 3).
Surgical technique
All patients were operated under general hypotensive anesthesia in a supine position with the head of the table slightly elevated (anti-Trendlenberg position). The head of the patient was rotated to the opposite side of the operated ear and supported with a head ring. The procedure was performed using a rigid, 2.7-mm diameter, 18-cm length 0° endoscope. The external auditory canal was injected with diluted adrenalin (1:200,000) using an insulin syringe distal to the hairy area throughout the circumference till the skin began to blanch. The edges of the perforation were freshened using a needle. A tympanomeatal flap was elevated, starting 0.5 cm lateral to the annulus from 12 o’clock to 6 o’clock, until reaching the tympanic annulus, which was then elevated with the remnant of the tympanic membrane and reflected anteriorly. The PRF membrane was placed in an underlay fashion medial to the handle of the malleus, and the middle ear was packed with gel foam to support the graft. The tympanomeatal flap was repositioned back and also supported with gel foam. The external auditory canal was packed with a piece of sterile gauze soaked in antibiotic ointment. A light ear dressing was performed.
Patients were discharged home the same day on systemic antibiotics and nasal decongestant with postoperative instructions to avoid strenuous physical activity or straining. The ear dressing and ear pack were removed 5 days later. Follow-up by endoscopic examination was made 2 weeks, 1 month, and 3 months postoperatively regarding graft take. The graft was considered successful if a complete seal of the tympanic membrane perforation was obtained with no residual perforation. Audiological assessment by pure tone audiometry was done 3 months postoperatively with the calculation of the air-bone gap as the mean of 500, 1000, and 2000 Hz thresholds.
Statistical analysis
The statistical package for the Social Sciences (SPSS) version 25 (IBM Corp., Armonk, NY, USA) was used to code and enter the data. For quantitative data, the mean, standard deviation, median, minimum, and maximum were used to summarize the data; for categorical data, the frequency (count) and relative frequency (percentage) were used. The non-parametric Mann-Whitney test was used to compare quantitative variables. We used the chi-square (χ2) test to compare categorical data. When the expected frequency was less than 5, an exact test was utilized instead. Statistical significance was defined as P values of less than 0.05.
Results
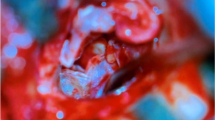
The study included 20 patients, 8 males and 12 females, with an age range of 15–45 years (mean 23.95 ± SD 8.01). All patients had conductive hearing loss with an air-bone gap ranging from 5 to 30 dB, mean of 18 ± SD 8.94. In the 2nd post-operative week, the graft was in place in 16 patients (80%), while there was residual perforation in 1 patient (5%) and complete loss of graft in 3 patients (15%). One month postoperatively, successful repair was achieved in 12 patients (60%) (Fig. 4), while there was residual perforation in 2 patients (10%) (Fig. 5), and complete loss of graft in 6 patients (30%). Patients developed no hearing loss, tinnitus, vertigo, bleeding, taste disturbance, or hyperkeratosis. Only one case developed external auditory canal otomycosis that was dealt with successfully with topical antifungal cream with no affection of graft take. The mean air-bone gap improved from 18 ± 8.94 dB preoperatively to 14.5 ± 7.93 dB postoperatively (P value > 0.05).
Discussion
Eardrum perforations are among the most common indications for otological surgery. Myringoplasty is a surgical procedure that is confined to the ear drum without manipulation of the ossicles or middle ear [1].
Surgeons worldwide have made accidental and intentional contributions to find the perfect graft material and the best approach to perform tympanoplasty. The two most commonly used materials are temporalis fascia and cartilage [1].
Choukroun et al. first introduced PRF as a second-generation platelet concentrate without the use of anti-coagulants. It was developed with shorter preparation times [11]. PRF fulfills all three essential requirements for tissue regeneration: it serves as a three-dimensional fibrin scaffold; it contains autologous cells like neutrophils, platelets, leukocytes, and macrophages; and it acts as a reservoir for natural growth factors that can be released over a 10- to 14-day period [10].
PRF usage in the repair of chronic tympanic membrane perforation has also taken place in the last decade by Braccini et al. It is used in combination with graft material “hamburger technique” or alone in the form of a cylinder that seals the perforation “champagne plug technique” [12].
El-Anwar et al. used PRF as an autologous graft in repairing small tympanic membrane perforations with satisfactory results regarding graft take and no postoperative infection [13].
Nair et al. studied the safety and efficacy of PRF in myringoplasty. He performed underlay myringoplasty using a temporalis fascia graft. PRF was placed as a plug over the sealed perforation. This procedure yielded satisfactory results regarding graft take and less postoperative infection [14].
Our current study included 20 patients with dry central tympanic membrane perforations. The patients underwent endoscopic myringoplasty using PRF only by underlay technique. The aim of the study was to evaluate the safety and efficacy of PRF alone regarding graft take rate and hearing improvement.
In our study, 60% of the patients had a completely healed tympanic membrane postoperatively, 10% of the patients had a partially (nearly 50%) healed tympanic membrane postoperatively, and 30% of the patients had complete loss of the graft at the end of the follow-up period (mean follow-up period was 3 months).
Compared with other studies, Habesoglu et al. used PRF on 14 patients with acute traumatic tympanic membrane perforation with a success rate of 64.3% [15]. Ozer et al. used PRF graft in an onlay fashion to repair traumatic tympanic membrane perforation in 30 patients with a graft take rate of 93% [16]. Braccini et al. used PRF in the form of a champagne plug technique in small perforations with a success rate of 96% [12].
Regarding hearing outcome in our study, the mean preoperative air-bone gap was 18 dB, the mean 3-month postoperative air-bone gap was 14.5 dB, and the mean air-bone gap closure was 3.5 dB. Our results were comparable but less than those of other studies. This could be due to the relatively short follow-up period in our study, where pure tone audiometry was performed 3 months postoperatively, while in most studies, it was performed 6 months postoperatively.
Compared with other studies, Ozer et al. reported hearing improvement in the mean air-bone gap of 14.1 dB [16]. El-Anwar et al. found improvement in the mean air-bone gap at speech frequencies from 16 ± 3.83 dB preoperatively to 7 ± 2.9 dB postoperatively [13].
Advantages of PRF usage in myringoplasty include its ease of preparation and handling, as well as the absence of side effects. It is cheap and easily available. Platelet-rich plasma contains a high concentration of white blood cells, which helps to prevent infection by functioning as a bactericidal agent. These platelets have an in vivo half-life of around 7 to 10 days, which is enough time for the healing process [13, 14]. Advantages also included the avoidance of any skin incisions (postauricular or endaural), resulting in less operative time, less postoperative pain and morbidity, sooner recovery, and a better cosmetic outcome.
The limitations of our study are the small sample size and the relatively short duration of follow-up. Increasing the sample size and extending the follow-up period to 1 year would enhance the credibility of the study’s results.
Conclusions
Endoscopic myringoplasty using PRF alone can be done in cases with tympanic membrane perforations as an alternative to using other autologous grafts. It is a new reliable safe method for myringoplasty, as well as being an easy, time-saving procedure with less postoperative pain and morbidity.
Availability of data and materials
The datasets used during the current study are available from the corresponding author on reasonable request.
Abbreviations
- PRF:
-
Platelet-rich fibrin
References
Sarkar S (2013) A review on the history of tympanoplasty. Indian J Otolaryngol Head Neck Surg 65(Suppl 3):455–460
Mudry A (2005) History of tympanoplasty. Otol Neurotol 26(3):554–555
Gersdorff M, Gerard JM, Thill MP (2003) Overlay versus underlay tympanoplasty. Comparative study of 122 cases. Rev Laryngol Otol Rhinol 124(1):15
El-Sheikh MM (2019) Evaluation of hearing outcome of tympanoplasty using cartilage graft versus temporalis fascia graft. Egypt J Otolaryngol 35(1):1
Dursun E, Dogru S, Gungor A, Cincik H, Poyrazoglu E, Ozdemir T (2008) Comparison of paper-patch, fat, and perichondrium myringoplasty in repair of small tympanic membrane perforations. Otolaryngol Head Neck Surg 138(3):353–356
Ozgursoy OB, Yorulmaz I (2005) Fat graft myringoplasty: a cost-effective but underused procedure. J Laryngol Otol 119(4):277–279
DiLeo MD, Amedee RG (1996) Fibrin-glue-reinforced paper patch myringoplasty of large persistent tympanic membrane perforations in the guinea pig. ORL 58(1):27–31
Choukroun J, Diss A, Simonpieri A, Girard MO, Schoeffler C, Dohan SL, Dohan AJ, Mouhyi J, Dohan DM (2006) Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part IV: clinical effects on tissue healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol 101(3):e56–e60
Dohan Ehrenfest DM, Del Corso M, Diss A, Mouhyi J, Charrier JB (2010) Three-dimensional architecture and cell composition of a Choukroun’s platelet-rich fibrin clot and membrane. J Periodontol 81(4):546–55
Miron RJ, Choukroun J (2017) Platelet rich fibrin a second generation platelet concentrate. First edition: platelet rich fibrin in regenerative dentistry: biological background and clinical indications. Wiley, New Delhi, pp 1–5. Ch 1
Choukroun J, Adda F, Schoeffler C, Vervelle APRF (2001) Une opportunité en paro-implantologie: le PRF. Implantodontie 42(55):e62
Braccini F, Tardivet L, Dohan DE (2009) The relevance of Choukroun’s Platelet-Rich Fibrin (PRF) during middle ear surgery: preliminary results. Rev Laryngol Otol Rhinol 130(3):175–180
El-Anwar MW, Elnashar I, Foad YA (2017) Platelet-rich plasma myringoplasty: a new office procedure for the repair of small tympanic membrane perforations. Ear Nose Throat J 96(8):312–326
Nair NP, Alexander A, Abhishekh B, Hegde JS, Ganesan S, Saxena SK (2019) Safety and efficacy of autologous platelet-rich fibrin on graft uptake in myringoplasty: a randomized controlled trial. Int Arch Otorhinolaryngol 23(01):077–082
Habesoglu M, Oysu C, Sahin S, Sahin-Yilmaz A, Korkmaz D, Tosun A, Karaaslan A (2014) Platelet-rich fibrin plays a role on healing of acute-traumatic ear drum perforation. J Craniofac Surg 25(6):2056–2058
Gür ÖE, Ensari N, Öztürk MT, Boztepe OF, Gün T, Selçuk ÖT, Renda L (2016) Use of a platelet-rich fibrin membrane to repair traumatic tympanic membrane perforations: a comparative study. Acta Otolaryngol 136(10):1017–1023
Acknowledgements
Not applicable.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
MH had the idea of the work and contributed to the revision of the article. MHH performed some of the operations and contributed to the revision of the article. HOI analyzed and interpreted the patient data regarding the surgical procedure and the follow-up and helped in the scientific writing of the manuscript. MSS performed some of the operations and was a major contributor to the scientific writing of the manuscript (corresponding author). All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Institutional Review Board at Cairo University Hospitals on 19 March 2019 (Registration number 87-2019). Informed written consent to participate in the study was signed by all patients and by parents/guardians of patients under 16 years old.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hammouda, M., Heiba, M.H., Ibrahim, H.O. et al. Endoscopic transcanal underlay myringoplasty using a platelet-rich fibrin (PRF) membrane. Egypt J Otolaryngol 40, 50 (2024). https://doi.org/10.1186/s43163-024-00615-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43163-024-00615-3