Abstract
Background
The molecular makeup of a head and neck squamous cell carcinoma (HNSCC) could vary as per the geography and corresponding variability in prevalent etiopathological factors. The objective of this study was to analyze the expression pattern of E-cadherin (E-cad), a transmembrane glycoprotein with tumour suppressor function, in a cohort of HNSCC treated at a tertiary care medical centre in the southern part of India.
Material and methods
After obtaining the institutional ethics committee’s permission, the expression of E-cad in HNSCC was assessed by using immunohistochemistry on retrospectively collected tumour specimens, obtained by a surgical cohort of cases operated between September 2018 and July 2019. The E-cad expression was then correlated with various clinical and pathological characteristics of HNSCC, retrieved via the medical records of corresponding patients.
Results
A total of 60 patients of HNSCC were included, most of whom had lesion in the oral cavity, in an advanced stage. The majority had a strong or moderate expression of E-cad on the surface. On analyzing further, oral cavity tumours had significantly less expression of E-cad compared to laryngeal and hypopharyngeal tumours taken together, and primary tumours had less E-cad expression than recurrent cases. Multivariate analysis with proportional odds regression showed the significant associations of low expression of E-cad expression with the moderate/poor differentiation of tumours and with the extranodal extension.
Conclusions
Among the HNSCC, the loss of E-cad expression was mostly associated with primary tumours of the oral cavity, moderate/poorly differentiated tumours, and in those HNSCCs that had an extranodal extension.
Similar content being viewed by others
Background
Head and neck squamous cell carcinoma (HNSCC) is a group of heterogeneous tumours, belonging to various sites of the upper aerodigestive tract, and exhibiting distinct molecular and biological behaviour [1, 2]. It continues to be one of the major cancers in the developing world and adds significantly to the economic burden in the affected region [3, 4]. While the majority of HNSCC in the developed world are Human papilloma virus (HPV) related oropharyngeal cancers, most HNSCC in the developing region involve the oral cavity, larynx, and laryngopharynx, and are mostly related to tobacco intake [3,4,5]. There exists a vast variability between these tumours with respect to their aggressiveness and their therapeutic response [5]. Actually, the therapeutic response of a tumour is dependent on its pathological characteristics, and one of the crucial pathological determinants is the molecular makeup of the tumour [6,7,8,9]. Although the molecular signature of HNSCC has been landscaped recently, only a few of the molecular determinants have found a plausible practical utility [2, 8, 10]. One such molecular marker with potential clinical application in early diagnosis and predicting the prognosis of HNSCC is E-cadherin (E-cad) [11, 12].
E-cad is a transmembrane glycoprotein belonging to a large family of calcium-dependent cell adhesion molecules called cadherin. It plays a crucial role in the formation and maintenance of cell-to-cell adhesion junctions among the epithelial cells [13]. E-cad, acting as tumour suppressor protein, is expressed abundantly on the normal cell membrane, but its expression progressively reduces through the phases of epithelial dysplasia, carcinoma-in-situ and invasive cancer [14]. The loss of membrane expression of E-cad is said to enhance the invasiveness of tumour by inhibiting cellular adhesion, differentiation, and apoptotic ability [13]. Interestingly, the loss of membranous E-cad has also been linked to increased expression of cytoplasmic E-cad, but the practical implication of the latter needs further exploration [15]. Nevertheless, the loss of membrane E-cad expression reflects the aggressive nature of the tumour and has shown to have a significant association with reduced survival in HNSCC [13,14,15,16,17]. However, the molecular makeup of a tumour could vary as per the geography and corresponding variability in the prevalence of etiopathological factors. With the intention of understanding the molecular heterogeneity of E-cad in HNSCC in this part of the world, we evaluated the expression of E-cad in a cohort of surgically treated HNSCC.
Methods
Study design
This single-centre exploratory study was conducted by the Department of Otolaryngology-Head and Neck Surgery in collaboration with the Department of Pathology, of a tertiary care hospital and cancer centre located in the southern part of India. The primary objective of the study was to evaluate the tumour expression of E-cad in patients with HNSCC and correlate the expression pattern with various clinicopathological factors.
Subjects
All the biopsy-proven cases of HNSCC, either primary or recurrent, who had undergone a curative intent surgical treatment at our centre between Sept 2018 and July 2019 were evaluated for possible inclusion into the study. Exclusion criteria consisted of patients with previous non-head and neck malignancy, incomplete medical records, and those cases in whom further pathological processing to study the E-cad expression was not possible.
Collection of data
The required clinical details of the included patients were retrieved from the medical record department. Demographical characteristics such as age and gender, and the tumour-related clinicopathological findings such as the site, size, stage, and status (whether primary or recurrent) were tabulated in an Excel sheet. The postoperative pathological report of each case was reviewed for information like pathological tumour staging, WHO grading of tumour differentiation, lymphovascular invasion (LVI), metastatic disease in excised lymph nodes, and extranodal extension (ENE). Using these details, the tumour stage and nodal stage as per the 8th edition of the American Joint Committee on Cancer staging manual were assigned (verified) to all the included subjects. The included patients’ primary tumour specimens were further processed for assessing the expression pattern of E-cad on the surface of the tumour.
Processing of pathological specimen
In the Department of Pathology, 10% neutral buffered formalin was used for the fixation of tissue specimens. All tissues were submitted and processed overnight in an automated tissue processor according to standard protocols. The paraplast blocks were prepared with standard embedding protocols of the department and the slides were then stained with Eosin and Hematoxylin counterstain. E-cad immunohistochemistry (IHC) was performed as per standard protocol and assessed with a semi-quantitative scoring system ranging from 0 to 3 + . A score of 0 represents the absence of staining, 1 + represents weak membranous staining, 2 + represents intermediate intensity of membrane staining, and 3 + reflects strong intensity of membrane staining. Staining in normal squamous epithelium was considered as positive control. Tissue stained without secondary antibody constituted a negative control.
Outcome analysis
The expression pattern of E-cad in the overall study cohort of HNSCC was analysed as per the staining characteristics on IHC. We also analysed the association of E-cad expression with the predictive variables such as age, gender, pathological T stage, LVI, pathological nodal status, and ENE. A subgroup analysis was carried out for the sub-set with oral cavity tumours.
Statistical analysis
SPSS version 20.0 was used for all the statistical analysis. The association between the E-cad expression and the various clinicopathological factors were evaluated by univariate and multivariate analyses. For univariate analysis, we used Kruskal–Wallis test, and for multivariate analysis, we performed proportional odds regression to see if any of these factors could independently predict the E-cad expression pattern. A p value of < 0.05 was considered statistically significant in all these analyses.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. The approval was granted by the Ethics Committee of Kasturba Medical College (Date: Aug. 14, 2019, IEC number: 581/2019).
Results
Demographics and clinicopathological characteristics
After screening the patients operated during the study period, a total of 67 patients were deemed eligible for inclusion into the study. However, as few patients were excluded due to incomplete medical records (n = 2) and lack of pathological details (including those in whom E-cad expression couldn`t be assessed) (n = 5), the total sample size included for analysis was 60. The mean age of the included patients was 55 years, with a range of 22 to 78 years. Interestingly, the majority of the included patients were men (n = 46) with M:F ratio of 3.3:1. Among our operated cohort of HNSCC, the epicentre of the tumour was alveolus in 8, retromolar trigone in 2, buccal mucosa in 13, oral tongue in 25, larynx in 8, and hypopharynx in 4. 51 of 60 patients had undergone surgery for primary tumours, and remaining nine were operated for recurrent or residual diseases. While most of the primary tumours belonged to the oral cavity (44/51; 86.3%), the recurrent tumour group contained mostly the locally advanced laryngeal and hypopharyngeal tumours (5/9; 55.5%) undergoing salvage total laryngectomy with or without total pharyngoesophagectomy. Those patients undergoing salvage laryngectomy had either recurred (n = 3) or progressed (n = 1) with cisplatin-based concurrent chemoradiation or recurred following laser excision (n = 1), and those with recurrent oral cancer had received previous surgical treatment with (n = 1) or without adjuvant treatment (n = 3). With respect to the pathological stage of the excised tumour, we noted T1 tumours in 6, T2 and T3 in 16 each, and T4 in 22 cases. Concerning tumour differentiation, 35 were G1 tumours (well-differentiated), 24 were G2 tumours (moderately differentiated), and one tumour was G3 in nature (poorly differentiated). Similarly, the excised tumour showed LVI in 31 cases, and the excised lymph nodes showed positive tumour deposits in 24, with ENE in 17 of those.
Tumour E-cad expression and its association with clinicopathological predictors
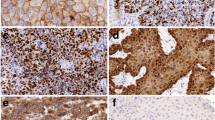
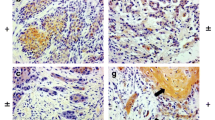
The E-cad expression on the tumour tissue identified by the IHC showed intermediate intensity of membrane staining (diffuse 2 +) in 24 and strong intensity of membrane staining (diffuse 3 +) in 29 tumour blocks. The membrane staining was weakly intense (diffuse 1 +) in 2 of the tumour blocks and was not appreciable (0 or negative) in the remaining 5. Figure 1 demonstrates the various staining intensities of E-cad expression in our study cohort. On analysing the relationship between the E-cad expression pattern and various clinicopathological variables, as depicted in Table 1, it was noticed that the oral cavity tumours had significantly less expression of E-cad compared to laryngeal and hypopharyngeal tumours taken together. Similarly, the primary tumours were associated with lower E-cad expression compared to recurrent/residual tumours. Interestingly, there was a clear trend of increased expression of E-cad with increased pT stage; however, the difference was not statistically significant. The E-cad expression did not differ significantly with the tumour differentiation, LVI, lymph node status, and ENE during the univariate analysis. However, multivariate analysis with proportional odds regression showed significant associations of low expression of E-cad expression with the moderate/poor differentiation of tumours and with the presence of ENE. On subgroup analysis of the oral cavity tumours, as shown in Table 2, low E-cad expression was associated significantly with the tumours involving the tongue on univariate analysis, and with the moderately/poorly differentiated tumours on both univariate and multivariate analyses.
Photomicrographs showing immunohistochemistry results of membrane expression of E-cad, in specimens of head and neck squamous cell carcinoma. A Haematoxylin-rich nuclei with non-visualisation of the cell membrane due to absence of E-cad expression on the surface, representing a score of 0; B Weakly stained (brown in colour) cell membrane with counter-stained nuclei, representing a score of 1 + ; C Intermediate intensity of cell membrane staining (brown hue in-between weak and strong), taken as a score of 2 + ; and D Strongly stained cell membrane (intense dark brown) reflecting a score of 3 + . All 4 photomicrographs are magnified 400 times (× 400)
Discussion
This study analysed the E-cad expression on the surface of resected HNSCC specimens collected over a year. Our surgical cohort mostly contained men and predominantly consisted of primary tumours of the oral cavity with some recurrent/residual tumours of the larynx and hypopharynx. However, the pattern of E-cad expression was not affected by the gender or age in our study. With respect to the whole surgical cohort of HNSCC, more than 50% of the studied tumour specimens exhibited less than the moderate expression of E-cad. The most apparent association of E-cad expression pattern in our study was noted with the grade of tumour differentiation. The immunoexpression of E-cad on tumour surface clearly reduced with the loss of cellular differentiation, indirectly supporting the prevalent hypothesis about the loss of E-cad expression in aggressively behaving poorly differentiated tumours [18,19,20,21]. In fact, in our study, the increased grading of cellular differentiation was an independent predictor of lower or loss of E-cad expression in all HNSCC combined, as well as in sub-cohort of oral cavity tumours.
Among the various other clinicopathological factors studied, the site of the tumour had a significant correlation with E-cad expression on univariate analysis. Compared to tumours of laryngeal and hypopharyngeal origin, tumours arising from the oral cavity had significantly less expression of E-cad suggesting their relative aggressiveness. Many previous studies have reported reduced immunoexpression of E-cad in up to 40–50% of oral cavity cancers [14, 20, 22]. Although we did not do a survival analysis in our study, loss of E-cad expression has been directly linked to reduced survival in oral cavity tumours in other studies [23]. Interestingly, the association of E-cad expression with aggressive laryngeal tumours and their survival outcomes has not been compelling in the literature, as the studies have reported contradicting results [24,25,26,27]. While some of the previous studies have shown the reduced immunoexpression of E-cad even in laryngeal lesions, our results did not concord with the literature, which probably has to do with the number and nature of laryngeal cancers included in the present study [25, 26].
Most of the tumours larynx/hypopharynx in our study had received prior chemotherapy along with the radical radiation as the primary therapeutic modality, and the E-cad expression in these patients was studied on their surgical specimen after the salvage laryngectomy/pharyngectomy. In other words, compared to most of the tumours of the oral cavity, which were primary in nature, a significant proportion of laryngeal tumours included in our study were recurrent. Also, the laryngeal tumours were disproportionately less in number as opposed to the oral cavity tumours. Considering these disparities, and the fact that the recurrent laryngeal tumours in our series had strong expression of E-cad on their surface, one could presume a plausible role of chemoradiation in restoring the expression of E-cad on the membranous of these tumours. There are reports of increased E-cad expression in HPV-16-positive cell lines of HNSCC treated with certain chemotherapeutics like tyrosine kinase inhibitors in vitro [28].
On multivariate analysis, the only other independent predictor of low E-cad expression in the overall cohort of surgically treated HNSCC was the presence of ENE. However, the presence of the metastatic lymph node itself did not have any bearing on E-cad expression pattern in our study. Although many studies in the past have found a positive association between low E-cad expression and the presence of cervical lymph node metastasis, there are a few who have reported the absence of such an association [18, 29]. Our results are in agreement with the latter. Interestingly, we could not find any previous study that has analysed/reported the association between the E-cad expression and ENE in HNSCC. To our knowledge, ours is the first to have found a positive association between the presence of ENE and low E-cad expression, supporting the notion of aggressiveness that surrounds the loss of E-cad expression.
Considering the uneven distribution of tumours as per the sites, a separate analysis was done for the surgical cohort of oral cavity tumours. In this sub-group analysis, the tumours of the oral tongue had significant loss of E-cad expression on their surface compared to the other sites of the oral cavity, reflecting the relatively aggressive behaviour of tongue lesions. As seen in overall study cohort, even during the separate analysis of the resected oral cavity tumour specimens, the moderate/poor differentiation group showed significant association with the low E-cad expression compared to the group of well-differentiated tumours. A similar study on E-cad expression in oral cavity tumours from the sub-continent has found a significant association between the E-cad expression and positive lymph node metastasis as well as with advanced stage of the disease; however, it did not find the variation in E-cad expression as per the differentiation of the tumour [30]. Nevertheless, most of these previous reports are in contrast to our results, highlighting the heterogeneity in the tumour biology and corresponding molecular alterations. Also, the tumour site, as well as the methodology (especially with respect to measurement and analysis of E-cad expression) were different between studies, although, that alone would not be sufficient to explain the gross disparities in the results. Similar inconsistencies between the studies have been identified by the reviews on E-cad expression, with respect to tumour size, nodal stage, tumour differentiation, and even with its impact on survival, which could be attributed to gross heterogeneity between the methodology of the studies and partly to the probable variability in E-cad expression itself [12, 15, 26]. Nevertheless, larger studies in future, preferably prospective in nature, may help in further characterising the E-cad expression pattern in this part of the world that has the highest prevalence of tobacco-related HNSCC. Apart from the disproportionate representations between the laryngeal and oral cavity tumours, as well as between the primary and recurrent tumours, the other limitations of our study are its retrospective nature and the lack of follow-up data or survival analysis.
Conclusion
In our study, loss of E-cad expression was found to have a significant association with the loss of cellular differentiation in HNSCC, in general, as well as in a subgroup of primary oral cavity cancers. Also, the presence of ENE was associated with the loss of E-cad expression in our study, in line with the negative prognostic value of ENE, as well as that of loss of E-cad expression on the membrane. Comparatively, more of the resected oral cavity tumours had reduced expression of E-cad on their surfaces, reflecting their aggressive behaviour as against the laryngeal counterparts; however, most of the latter cases were salvage resections for failed chemoradiation. Based on our results, we hypothesise that the cisplatin-based chemotherapy could restore E-cad expression on the surface of laryngeal/hypopharyngeal tumours.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
References
Jou A, Hess J (2017) Epidemiology and molecular biology of head and neck cancer. Oncol Res Trea 40:328–332
Beck TN, Golemis EA (2016) Genomic insights into head and neck cancer. Cancers of the Head & Neck 1. https://doi.org/10.1186/s41199-016-0003-z
Bray F, Ferlay J, Soerjomataram I et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424. https://doi.org/10.3322/caac.21492
Chaturvedi AK, Anderson WF, Lortet-Tieulent J et al (2013) Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol 31:4550–4559. https://doi.org/10.1200/JCO.2013.50.3870
Devaraja K, Aggarwal S, Verma SS, Gupta SC (2020) Clinico-pathological peculiarities of human papilloma virus driven head and neck squamous cell carcinoma: a comprehensive update. Life Sci 245:117383. https://doi.org/10.1016/j.lfs.2020.117383
Cho J, Johnson DE, Grandis JR (2018) Therapeutic implications of the genetic landscape of head and neck cancer. Semin Radiat Oncol 28:2–11
Sun W, Califano JA (2014) Sequencing the head and neck cancer genome: implications for therapy. Ann N Y Acad Sci 1333:33–42. https://doi.org/10.1111/nyas.12599
Devaraja K (2019) Current prospects of molecular therapeutics in head and neck squamous cell carcinoma. Pharmaceut Med 33:269–289. https://doi.org/10.1007/s40290-019-00288-x
Devaraja K, Aggarwal S, Singh M (2023) Therapeutic vaccination in head and neck squamous cell carcinoma-a review. Vaccines (Basel) 11:634. https://doi.org/10.3390/vaccines11030634
Aggarwal S, Devaraja K, Sharma SC, Das SN (2014) Expression of vascular endothelial growth factor (VEGF) in patients with oral squamous cell carcinoma and its clinical significance. Clin Chim Acta 436:35–40. https://doi.org/10.1016/j.cca.2014.04.027
Hussein AA, Forouzanfar T, Bloemena E et al (2018) A review of the most promising biomarkers for early diagnosis and prognosis prediction of tongue squamous cell carcinoma. Br J Cancer 119:724–736. https://doi.org/10.1038/s41416-018-0233-4
Ren X, Wang J, Lin X, Wang X (2016) E-cadherin expression and prognosis of head and neck squamous cell carcinoma: evidence from 19 published investigations. Onco Targets Ther 9:2447–2453. https://doi.org/10.2147/OTT.S98577
Wong SHM, Fang CM, Chuah LH et al (2018) E-cadherin: Its dysregulation in carcinogenesis and clinical implications. Crit Rev Oncol Hematol 121:11–22
Cheng S-Y, Shi K, Bai X-R et al (2018) Double-staining of E-cadherin and podoplanin offer help in the pathological diagnosis of indecisive early-invasive oral squamous cell carcinoma. Int J Clin Exp Pathol 11:38–47
Yazdani J, Ghavimi MA, Jabbari Hagh E, Ahmadpour F (2018) The role of E-cadherin as a prognostic biomarker in head and neck squamous carcinoma: a systematic review and meta-analysis. Mol Diagn Ther 22:523–535
Liu LK, Jiang XY, Zhou XX et al (2010) Upregulation of vimentin and aberrant expression of E-cadherin/Β-catenin complex in oral squamous cell carcinomas: Correlation with the clinicopathological features and patient outcome. Mod Pathol 23:213–224. https://doi.org/10.1038/modpathol.2009.160
Dumitru CS, Ceausu AR, Comsa S, Raica M (2022) Loss of E-cadherin expression correlates with Ki-67 in head and neck squamous cell carcinoma. In Vivo 36:1150–1154. https://doi.org/10.21873/invivo.12814
Schipper JH, Unger A, Jahnke K (1994) E-cadherin as a functional marker of the differentiation and invasiveness of squamous cell carcinoma of the head and neck. Clin Otolaryngol Allied Sci 19:381–384. https://doi.org/10.1111/j.1365-2273.1994.tb01252.x
Diniz-Freitas M, García-Caballero T, Antúnez-López J et al (2006) Reduced E-cadherin expression is an indicator of unfavourable prognosis in oral squamous cell carcinoma. Oral Oncol 42:190–200. https://doi.org/10.1016/j.oraloncology.2005.07.010
Angadi PV, Patil PV, Angadi V et al (2016) Immunoexpression of epithelial mesenchymal transition proteins E-cadherin, β-catenin, and N-cadherin in oral squamous cell carcinoma. Int J Surg Pathol 24:696–703. https://doi.org/10.1177/1066896916654763
Sharma J, Bhargava M, Aggarwal S et al (2022) Immunohistochemical evaluation of E-cadherin in oral epithelial dysplasia and squamous cell carcinoma. Indian J Pathol Microbiol 65:755–760. https://doi.org/10.4103/ijpm.ijpm_31_21
Segura I-G, Secchi D-G, Galíndez M-F et al (2022) Connexin 43, Bcl-2, Bax, Ki67, and E-cadherin patterns in oral squamous cell carcinoma and its relationship with GJA1 rs12197797 C/G. Med Oral Patol Oral Cir Bucal 27:e366–e374. https://doi.org/10.4317/medoral.25298
Yao X, Sun S, Zhou X et al (2017) Clinicopathological significance of ZEB-1 and E-cadherin proteins in patients with oral cavity squamous cell carcinoma. Onco Targets Ther 10:781–790. https://doi.org/10.2147/OTT.S111920
Zou J, Yang H, Chen F et al (2010) Prognostic significance of fascin-1 and E-cadherin expression in laryngeal squamous cell carcinoma. Eur J Cancer Prev 19:11–17. https://doi.org/10.1097/CEJ.0b013e32832f9aa6
Nardi CE, Dedivitis RA, de Almeida RC et al (2018) The role of E-cadherin and β-catenin in laryngeal cancer. Oncotarget 9:30199–30209. https://doi.org/10.18632/oncotarget.25680
Re M, Gioacchini FM, Scarpa A et al (2018) The prognostic significance of E-cadherin expressioin laryngeal squamous-cell carcinoma: a systematic review. Acta Otorhinolaryngologica Ital 38:504–510. https://doi.org/10.14639/0392-100X-2106
Kucuk U, Ekmekci S, Talu CK et al (2023) Relationship of E-cadherin, Beta-catenin, N-cadherin, ZEB1 and αSMA as epithelial mesenchymal transition markers with prognostic factors in early and advanced stage laryngeal squamous cell carcinomas. Indian J Pathol Microbiol 66:237–245. https://doi.org/10.4103/ijpm.ijpm_530_21
Kramer B, Hock C, Schultz JD et al (2017) Impact of small molecules on β-catenin and E-cadherin expression in HPV16-positive and -negative squamous cell carcinomas. Anticancer Res 37:2845–2852. https://doi.org/10.21873/anticanres.11636
Bayram A, Yüce I, Çaʇli S et al (2015) Predictive value of E-cadherin and Ep-CAM in cervical lymph node metastasis of supraglottic larynx carcinoma. Am J Otolaryngol 36:736–740. https://doi.org/10.1016/j.amjoto.2015.08.006
Shergill K, Sen A, Pillai HJ (2018) Role of E-cadherin and cyclin D1 as predictive markers of aggression and clonal expansion in head and neck squamous cell carcinoma. J Korean Assoc Oral Maxillofac Surg 44:182–190. https://doi.org/10.5125/jkaoms.2018.44.4.182
Acknowledgements
Nil.
Funding
This study was supported by the intramural funding (institute seed money for faculty research) of Manipal Academy of Higher Education, Manipal, Karnataka, India.
Author information
Authors and Affiliations
Contributions
DK was involved in the conceptualization, data collection, analysis, interpretation, and manuscript writing. SP was involved in the conceptualization, data interpretation, and manuscript writing. MV performed the histological examination and immunohistochemistry of the slides and was a major contributor to writing the manuscript. GV was involved in the histological examination and immunohistochemistry, and in interpretation, and manuscript writing. KP was involved in data interpretation and manuscript writing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Kasturba Medical College, Manipal (Date: 14th Aug 2019, IEC number: 581/2019). Since it was a retrospective study, consent from the participants was waived.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Devaraja, K., Pillai, S., Valiathan, M. et al. E-cadherin expression pattern in head and neck squamous cell carcinoma and its association with clinico-pathological predictors. Egypt J Otolaryngol 39, 138 (2023). https://doi.org/10.1186/s43163-023-00503-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43163-023-00503-2