Abstract
Background
To evaluate the incidence of hearing loss in neonates in our secondary care hospital under pilot UNHS programme. To assess association between various risk factors and neonatal hearing loss.
Methods
Prospective, observational cohort study was done in a secondary level hospital in North India after ethical approval, for 1 year. Inclusion criteria are as follows: neonates born in hospital during study period, consenting to testing. Exclusion criteria are as follows: sick neonates, non-consenting parents. Neonates underwent TEOAE at 48 h of birth; those failing retested at 1 month. Neonates failing 2nd stage are tested after 3 months using BERA. Neonates were evaluated for the presence of maternal/neonatal high-risk factors.
Results
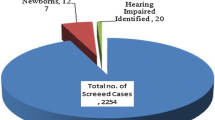
Out of 506 neonates, 143 passed 1st OAE screening, 363 were refer, and referral rate is 71.7%. A total of 341/345 neonates passed 2nd stage; 4 were diagnosed with hearing loss on BERA at 3 months. (18 neonates lost to follow-up, excluded from final cohort.) Overall incidence of hearing loss was 0.82%, 1.08% for males and 0.44% for females (p = 0.87, NS). One-hundred nine neonates were high risk (prematurity, 36; consanguinity, 4; caesarean section for relevant indications, 68; craniofacial abnormalities, 1). Incidence of hearing loss for high-risk group was 1.83% and 0.53% for well-born neonates (p = 0.19, NS).
Conclusion
Incidence of hearing loss in our district in North India is as follows: 8.2 per 1000 live births for well neonates, 18.3 per 1000 live births for high-risk neonates, and respective overall national incidence rates were 1.59 to 8.8 per 1000 and 7 to 49 per 1000. UNHS programmes must be implemented in all hospitals; protocol may be varied according to local population profile and resources available.
Similar content being viewed by others
Background
Hearing has a pivotal role in the evolution of normal speech and language. Lack of auditory stimulation leads to loss of speech and language development in the child (Northern and Downs, 1991). Along with hearing loss, there is loss of speech, vocabulary, literacy, academic skills, and lack of emotional and psychosocial development of the child [1]. Hearing loss is the second most prevalent cause of years lived with disability (YLD), which contributes to 4.7% YLD worldwide [2]. In South-East Asia region, hearing loss prevalence is 4.6 to 8.8%, while in India, it is 6.3% [2]. Hearing loss is a silent disorder; it normally comes to the parents’/caregivers’ knowledge only after about 2 years of age, by which time irreparable damage of language development has already occurred [3].
In 1994, the Joint Committee on Infant Hearing (JCIH) established screening of high-risk babies for hearing impairment using high-risk register (HRR) [4, 5]; however, screening high-risk neonates alone misses nearly 50% cases with hearing impairment. Thereafter in 1999, the American Academy of Paediatrics (AAP) endorsed the concept of Universal Hearing Screening Program (UNHSP) and remedial intervention. Universal neonatal hearing screening programme consists of two-tiered screening protocol in which initial assessment is done by OAE, and second screening is done with BERA [6, 7].
Rationale for our study is as follows: Universal Neonatal Hearing Screening (UNHS) is an essential goal of primary healthcare and an important component of any comprehensive otorhinolaryngology programme in secondary/tertiary hospitals. With the goal of starting UNHS in our hospital, we carried out the following study.
Review of literature
Studies done on implementation of universal new-born hearing screening (UHNS) over the last 15 years, published in indexed peer-reviewed journals, are summarised and presented in Table 1. Both national and international studies are included [8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24] (Table 1).
Verma et al. (2021) in their review found that the incidence of hearing loss in neonates ranged from 1.59 to 8.8 per 1000 live births. Among high-risk neonates. this number ranged from 7 to 49 per 1000 live births [31]. All included studies followed the 3-step screening protocol [31]. Boudewyns et al. (2020) performed a UNHS programme in their hospital and found that nearly 60% cases of bilateral sensorineural hearing loss were genetic. A study by Acke et al. (2021) found that 78% of new-borns referred to them had hearing loss. The most common cause was found to be otitis media with effusion (OME). The other causes were genetic, congenital cytomegalovirus infection, and external ear canal atresia [26].
A universal neonatal hearing screening (UNHS) programme needs to be cost-effective, or it cannot be sustained. A study carried out by Verkleij et al. (2021) found that the two-stage protocol was more cost-effective; the three-stage protocol led to a larger number of missed cases as parents did not report for follow-up screening visits. In the two-stage system, the first screening is carried out in the maternity ward itself, leading to high participation. Coming for a second visit thereafter results in greater compliance than having to come for two more subsequent visits. Algeria also being a third-world country, cost-effectiveness may mean the difference between a UNHS programme that is successful versus one that never takes off [29]. Similar findings were also reported by Kanji et al. (2018) who performed a meta-analysis of 15 studies on neonatal hearing screening programmes [27].
To conclude, most studies, whether national or international, unequivocally recommend implementation of universal new-born hearing screening. Sole high-risk group new-born hearing screening is very likely to miss many cases of congenital permanent hearing loss. This is another rationale for our study.
Aims and objectives
-
1.
To evaluate the incidence of hearing loss among new-borns in Panchkula (Haryana, India) and surrounding areas
-
2.
To assess the association between various risk factors and neonatal hearing loss
Methods
-
Ethical approval number: BREC/20/217. A prospective observational cohort study was carried out in the Department of Otorhinolaryngology, Civil Hospital, Sector-6, Panchkula Haryana 134,109. Ethical approval for the study was obtained from the Biomedical Research Ethics Committee, Pt. B.D. Sharma Post Graduate Institute of Medical Sciences, Rohtak.
-
Place of study: Department of Otorhinolaryngology, Civil Hospital, Sector-6 Panchkula, Haryana
-
Study design: Prospective, observational cohort study
-
Duration of study: One year
-
Study population: All neonates born at Civil Hospital, Sector-6 Panchkula
-
Sample size: Five-hundred consecutive neonates born during the study period in Civil Hospital, Sector 6 Panchkula
-
Sample size calculation
Taking 95% as confidence interval and absolute error of 5%, we took 0.5% (5 per 1000 live birth) prevalence as per research work by various Indian studies mentioned in “Review of literature”; thus, minimum sample size came to about 400. Taking into account the loss to follow-up, the total sample for the study was taken as 500.
P = 0.5 (expected prevalence from previous studies).
C = Acceptable margin of error for proportion being estimated.
Z = 1.96 for 95% (0.05) confidence level.
Study design and methodology
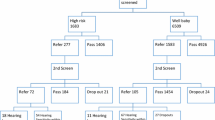
All new-born babies underwent hearing screening with transient-evoked otoacoustic emissions (TEOAE) followed by BERA according to the following protocol (Fig. 1).
Procedure of the test
Written informed consent was obtained from the mothers/parents/guardians. Information about the infant was recorded according to Performa in Annexure 1. All babies underwent routine ENT examination consisting of inspection of the pre-aural region, pinna, and post aural region. Occluding wax or debris was gently cleaned using cotton-tipped swab, and otoscopic examination of the tympanic membrane was carried out, followed by TEOAE done around 48 h after birth. Those who failed were tested subsequently after 1 month. Those cases who failed in both the tests were tested at 3 months using BERA.
OAE machine specifications: OAE screening unit
-
Make and model: Neurosoft Russia, Neuro-Audio Screen
-
TEOAE: Tone of 1–4 kHz, stimulus level 90-dB peak, Stimulus type: Non-linear click
BERA specifications
-
Make and model: Neurosoft Russia, Neuro Audio ABR with ASSR, 2 channels, and window based, click stimuli, recording of condensation-rarefaction stimuli, and insert earphones with new-born-size tip.
Inclusion criteria
-
1.
All neonates born in our hospital during study period
-
2.
All neonates whose parents/guardian gave consent to perform the test
Exclusion criteria
-
1.
Neonates who were too sick to undergo any kind of test, e.g. neonates with septicaemia, jaundice, systemic, or multi-organ failure (cardiac/renal/respiratory failure) and neonates on ventilator support.
-
2.
All neonates whose parents/guardians refused to give consent
All new-born babies were evaluated for the presence of the following high-risk criteria, and documentation was done of high-risk criteria if present:
-
High-risk criteria: Neonates were classified as high-risk (at high risk for congenital sensorineural hearing loss) if one or more of the following criteria were present: [9,10,11, 23, 24, 29].
Maternal risk factors
-
Family history of congenital or delayed onset childhood sensorineural hearing loss
-
Baby born out of consanguineous marriage
-
Maternal history of ototoxic drug intake
-
Maternal infections: Syphilis, HIV, hepatitis B, and TORCH
-
History of prolonged labour/perinatal asphyxia
Risk factors for new-born
-
Prematurity (< 37 weeks)
-
Birth weight < 1500
-
An illness or condition requiring admission of 48 h or more in NICU or special new-born care unit
-
APGAR score of 0–4 at first minute and 0–6 at 5 min
-
Septicaemia
-
Use of ototoxic drugs
-
Hyperbilirubinemia > 15 mg%
-
Stigmata or other findings associated with a syndrome known to include sensorineural hearing loss (e.g. Waardenburg’s or Usher syndromes)
-
The presence of craniofacial anomalies
Statistical analysis
All the relevant data was processed/compiled using Excel 2010 spreadsheet. For comparison of two groups which were normally distributed chi-square test was applied. Categorical variables have been reported in percentages and counts. Continuous data which is not being normally distributed has been reported as median and interquartile range. All analysis and graphical representation were done using SPSS Version 21(Statistical Package for The Social Science). p-value was set to 0.05 for significance.
Results
In our study, 506 consecutive neonates were included. Out of these, 143 passed the first OAE screening with 363 coming as refer. In the second-stage AOAE screening, only 345 reported for testing with 18 lost to follow-up. These 18 cases were removed from the final cohort. Out of the 345 who reported, 341 passed the second stage. Four babies came as refer. On undergoing diagnostic BERA, two (2 females) had moderate hearing loss, and two (both males, one with grade 2 anotia) had hearing loss of the moderate-moderately severe category. Thus, the incidence of hearing loss in our cohort of 488 (506—18 lost to follow-up) came out to be 0.82% (Table 2).
Sex distribution
In the final cohort of 488 babies, there were 264 male babies and 224 female babies. Incidence of hearing loss as distributed by sex was 1.08% for male neonates and 0.44% for female neonates. The difference was not statistically significant (p = 0.94) (Table 3).
Incidence of hearing loss in well babies versus high-risk babies
Out of all 488 neonates, 109 were high-risk (prematurity—36/consanguinity 4, caesarean section for relevant indications 68, and craniofacial abnormalities 1). The pooled data on incidence of hearing loss in these subgroups is as per Table 4.
Distribution according to individual risk factors
As elaborated in the “Materials and methods” section, the following risk factors were assessed and documented:
-
(A)
Maternal risk factors
-
Family history of congenital or delayed onset childhood sensorineural hearing loss: NIL
-
Baby born out of consanguineous marriage: 0–4
-
Maternal history of ototoxic drug intake: NIL
-
Maternal infections: Syphilis, HIV, hepatitis B, and TORCH: NIL
-
History of prolonged labour/perinatal asphyxia: 29 (underwent LSCS)
-
-
(B)
Risk factors for new-born
-
Prematurity (< 37 weeks): 36
-
Birth weight < 1500 grams: NIL
-
An illness or condition requiring admission of 48 h or more in NICU or special new-born care unit: NIL
-
APGAR score of 0-4 at first minute and 0-6 at 5 min: NIL
-
Septicaemia: NIL
-
Use of ototoxic drugs: NIL
-
Hyperbilirubinemia > 15 mg%: NIL
-
Stigmata or other findings associated with a syndrome known to include sensorineural hearing loss (e.g. Waardenburg’s or Usher syndromes): NIL
-
The presence of craniofacial anomalies: 0-1 (anotia)
-
Elaboration on risk factors
-
(A)
Consanguinity: Four out of the 502 babies had a history of coming from consanguineous marriages. Three passed the first-stage OAE, and one baby who came as refer passed the second-stage OAE, so incidence of hearing loss in this sub-group was 0% compared to the incidence of hearing loss in the non-consanguineous group—0.8% (p = 0.86, not significant) (Table 4).
-
(B)
Mode of delivery: lower segment caesarean section: Forty-eight babies were delivered by lower-segment caesarean section, indications for which were meconium-stained liquor (MSL), in 19 cases or prolonged labour — seen in 29 cases. All 48 babies passed the first-stage OAE screening; hence, incidence of hearing loss in this sub-group was 0% (Table 4).
-
(C)
Prematurity: Thirty-six out of 488 neonates were delivered prematurely. All were delivered vaginally. Out of these, only 7 passed the first stage with 29 coming as refer. All 29 neonates underwent stage 2 testing; one male baby came as refer, and he was sent for further diagnostic BERA which confirmed moderate to moderately severe hearing loss. Hence, incidence of hearing loss in this sub-group was 2.7%. Compared with the full-term neonates, the difference in incidence of hearing loss was not significant (Table 4).
-
(D)
Craniofacial anomalies: Out of all 488 neonates, only one had anotia, and she was found to have moderate-moderately severe hearing loss.
Discussion
Screening for hearing loss is an essential tool in the armamentarium of the primary-care paediatrician and ENT specialist. It is the responsibility of every healthcare centre to be equipped with universal neonatal hearing screening (UNHS). The JCIH (Joint Committee on Infant Hearing) is the main reference document for planning and executing UNHS in hospitals/healthcare settings. The primary goal, updated in 2019, is screening by 1 month of age, diagnosis by 2 months of age, and intervention by 3 months of age. Intervention can be in the form of hearing aids, amplified speech, or cochlear implants. The minimum age of getting a cochlear implant has been steadily decreasing; the latest criterion is 6 to 9 months of age [32]. As remedies for hearing loss are available, the need and relevance of neonatal hearing screening become more pronounced.
Aetiology of neonatal hearing loss
The most common causes of neonatal hearing loss are genetic (50%), congenital cytomegalovirus and other infections (20%), and temporal bone abnormalities [26, 30] (30–40%). Genetic causes are further classified into nonsyndromic causes (DFNB1, DFNB 3, DFNB 16, DFNB 21 mutations, and mitochondrial hearing loss) and syndromic hearing loss [Pendred syndrome, Usher syndrome, Jervell and Lange-Nielsen syndrome (all autosomal recessive), Alport syndrome (X-linked recessive), Waardenburg syndrome (can be autosomal dominant or recessive), branchio-oto-renal syndrome (autosomal recessive), and CHARGE syndrome)]. Nonsyndromic hearing loss cases usually present with milder degrees of hearing loss and involve single-gene mutations. These may present later in life and hence be missed by neonatal hearing screening; hence, long and/or regular follow-up check-ups should also be done [26, 30, 31, 33].
-
Infections: TORCH infections (Toxoplasma gondii, Rubella, Cytomegalovirus, herpes, and congenital syphilis
-
Other causes: Birth asphyxia, low birth weight, hyperbilirubinemia, sepsis, administration of ototoxic drugs (to treat pneumonia and sepsis), meningitis, and prolonged ventilation [34, 35]
Our observations compared with the literature
Overall incidence of hearing loss in our study was 8.2 per 1000 live births. The incidence of hearing loss in well babies was 5.3 per 1000 live births, and the incidence for high-risk babies was 18.3 per 1000 live births. These numbers are comparable to Verma et al.’s respective figures (1.59 to 8.8 per 1000 live births, well neonates; 7 to 49 per 1000 live births, high-risk neonates). A similar study done in Brazil by Chiriboga et al. gave the incidences of hearing loss in their neonates as 0.04% or 0.4 per 1000 live births in the well-baby population and 0.24% or 2.4 per 1000 live births in the high-risk neonates [28]. This is less compared to the data from India.
Referral rates in our study
In our study, 143 neonates passed the first stage; hence, 363 were referred to undergo second-stage screening. The referral rate is 363/506 or 71.7%. Out of 363 referred neonates, only 345 showed up for the second-stage TEOAE screening. Therefore, the percentage of our study cohort lost to follow-up is 18/363 or 4.9%. Comparing the percentages with those found in the study by Hrncic et al., their referral rate was 19.1% (223/1217 neonates), and their loss to follow-up was 38.1% (85/223) [36]. Thus, our referral rate was quite high. Possible reasons for such a high referral rate may be as follows: In the early stages of life, as within 48 h of birth, the external and/or middle ear of the neonate may still contain fluid/amniotic fluid or vernix caseosa. Although full care was taken during examination of the neonates, which included a gentle mopping of the external auditory canal for any debris, the middle or external ear might have still contained some fluid or debris. Any kind of such factor would impair the recordings of the OAEs. Moreover, according to a study done by Dhawan et al. and Mathur et al., the external auditory canal of neonates is very collapsible in less than 48 h of life [8, 9]. This may also contribute to the false-positive referrals of neonates to the second-stage screening. Yet another reason could be that the room where the tests were carried out had high ambient noise.
Patients lost to follow-up
As mentioned above, our loss to follow-up was 4.9% (18/363). Compared to Hrncic et al.’s study, where the loss to follow-up was 19.1%, our loss was relatively low, in fact only 1/3rd of this figure [36]. This shows a quite good compliance on the part of the parents/guardians of our cohort. Hrncic et al. conducted telephonic interviews with parents to find out the reasons for their absence from the second stage of the study. They found the most common reasons to be as follows: distance from the hospital, lack of knowledge on the part of the parents/guardians regarding the importance of the screening tests, and simply that some parents forgot to attend the follow-up appointments [36]. Thus, there are many reasons for loss to follow-up.
What could be the reasons for our low figure of loss to follow-up?
Ours is a district hospital, and most patients were from the local and nearby areas. Thus, there was no issue of our patients coming from far-flung areas. Moreover, as our study was conducted on well babies only, the chances of neonates not following up due to illness was less than it would have been if the group included babies from SNCU (special neonatal care unit) as well. Thirdly, our hospital regularly conducts awareness drives in the local community regarding government primary prevention programmes. Our national programme for care and prevention of hearing loss is NPPCD (National Program for Prevention of Childhood Deafness). Under this programme, regular IEC (information, education, communication) activities are carried out in the community in the form of posters, talks etc. which might have also contributed a bit to awareness in the parents, of the importance of hearing screening. Our study protocol has used TEOAEs as the TEOAE is more sensitive in the detection of hearing loss. It is more time-consuming than DPOAEs, but as sensitivity to detect cases of hearing loss is higher, it is mostly the preferred modality. In our study protocol, we have been using TEOAE in both the first and second stages, which is similar to many protocols of UNHS used in various countries.
Limitations of our study
Limitations of our study are as follows: Relatively limited sample size of about 500 neonates, which may not have given a very accurate estimation of significance levels of the various sub-groups. Larger sample sizes lead to better estimates, lesser chance of type 1 and type 2 errors, and overall give a better picture if the difference in the incidences of hearing loss in the various subgroups is significant or not. Moreover, our sample did not include unwell babies such as those admitted in the SNCU (special neonatal care unit). Including such children would have led to inclusion of other risk factor sub-groups, such as babies suffering from hyperbilirubinemia and babies who received ototoxic antibiotics. This would have given us a wider picture of the incidence of hearing loss and led to a more comprehensive study. However, our study is valuable as it is the pilot study of the first UNHS ever implemented in our area. Regular implementation, audit of data, feedback, and further inclusion of every single baby born, including babies from intensive care, are required to make this pilot programme a success.
Conclusion
Success of UNHS is dependent on many factors; some are tangible and can be modified, while others are not so well-defined. All areas must implement some form of UNHS programmes according to the facilities and manpower available, availability of funds, and local community profile. Regular follow-up, audit of data, and monitoring are essential for the long-term success of any UNHS programme [35, 37,38,39,40].
Availability of data and materials
All data available on request.
References
Acke FRE, De Vriese C, Van Hoecke H, De Leenheer EMR (2022) Twelve years of neonatal hearing screening: audiological and etiological results. Eur Arch Otorhinolaryngol 279(7):3371–3378
Bachmann KR, Arvedson JC (1998) Early identification and intervention for children who are hearing impaired. Pediatrics 19(5):155–165
Blanař V, Škvrňáková J, Pellant A, Vodička J, Praisler J, Boháčová E, Dršata J, Šenkeřík M, Chrobok V (2021) Effectiveness of neonatal hearing screening system: a 12-year single centre study in the Czech Republic. J Pediatr Nurs 59:e32–e37
Boudewyns A, van den Ende J, Declau F, Wuyts W, Peeters N, Brandt AHD, Van Camp G (2020) Etiological work-up in referrals from neonatal hearing screening: 20 years of experience. Otol Neurotol 41(9):1240–1248
Chiriboga LF, Sideri KP, Ferraresi Rodrigues Figueiredo SN, Monteiro Pinto ES, Chiriboga Arteta LM (2021) Outcomes of a universal neonatal hearing screening program of 9941 newborns over a one-year period in Campinas, Brazil. Int J Pediatr Otorhinolaryngol 148:110839
Davis A, Bamford J, Stevens J (2001) Performance of neonatal and infant hearing screens: sensitivity and specificity. Br J Audiol 35(1):3–15
De Capua B, Costantini D, Martufi C, Latini G, Gentile M, De Felice C (2007) Universal neonatal hearing screening: the Siena (Italy) experience on 19,700 newborns. Early Human Dev 83(9):601–606 (Epub 2007 Feb 20)
John M, Balraj A, Kurien M (2009) Neonatal screening for hearing loss: pilot study from a tertiary care centre. Indian J Otolaryngol Head Neck Surg 61(1):23–26
Joint Committee on Infant Hearing; American Academy of Audiology; American Academy of Pediatrics; American Speech-Language-Hearing Association; Directors of Speech and Hearing Programs in State Health and Welfare Agencies. Year 2000 position statement: principles and guidelines for early hearing detection and intervention programs. Joint Committee on Infant Hearing, American Academy of Audiology, American Academy of Pediatrics, American Speech-Language-Hearing Association, and Directors of Speech and Hearing Programs in State Health and Welfare Agencies. Pediatrics. 2000 Oct;106(4):798–817.
Joint Committee on Infant Hearing 1994 Position Statement. American Academy of Pediatrics Joint Committee on Infant Hearing. Pediatrics. 1995;95(1):152–6.
Kanji A, Khoza-Shangase K, Moroe N (2018) Newborn hearing screening protocols and their outcomes: a systematic review. Int J Pediatr Otorhinolaryngol 115:104–109
Kaveh M, Mirjalali SN, Shariat M, Zarkesh MR (2021) Perinatal factors influencing the neonatal hearing screening results. BMC Pediatr 21(1):15
Korver AM, Smith RJ, Van Camp G, Schleiss MR, Bitner-Glindzicz MA, Lustig LR, Usami SI, Boudewyns AN (2017) Congenital hearing loss. Nat Rev Dis Primers 12(3):16094
Kumar A, Gupta SC, Sinha VR (2017) Universal hearing screening in new-borns using otoacoustic emissions and brainstem evoked response in eastern Uttar Pradesh. Indian J Otolaryngol Head Neck Surg 69(3):296–299
Lieu JEC, Kenna M, Anne S, Davidson L (2020) Hearing loss in children: a review. JAMA 324(21):2195–2205
Maqbool M, Najar BA, Gattoo I, Chowdhary J (2015) Screening for hearing impairment in high risk neonates: a hospital based study. J Clin Diag Res 9(6):18–21
Mathers C, Smith A, Concha M (2000) Global burden of hearing loss in the year 2000. Global burden of Disease 18(4):1–30
Mishra G, Sharma Y, Mehta K, Patel G (2013) Efficacy of Distortion Product Oto-Acoustic Emission (OAE)/auditory brainstem evoked response (ABR) protocols in universal neonatal hearing screening and detecting hearing loss in children <2 years of age. Indian J Otolaryngol Head Neck Surg 65(2):105–110
Mathur NN, Dhawan R (2007) An alternative strategy for universal infant hearing screening in tertiary hospitals with a high delivery rate, within a developing country, using transient evoked oto-acoustic emissions and brainstem evoked response audiometry. J Laryngol Otol 121(7):639–643
Nagapoornima, P., Ramesh, A., Srilakshmi, Rao, S., Patricia, P.L., Gore, M, Dominic, M, Swarnarekha. Universal hearing screening. Indian Journal of Pediatrics. 2007 Jun;74(6):545–9.
National Sample Survey Organisation. Disabled persons in India: NSS 58th Round, July-December 2002. National Sample Survey Organisation. Ministry of Statistics and Programme Implementation, Government of India; 2003.
Parab SR, Khan MM, Kulkarni S, Ghaisas V, Kulkarni P (2018) Neonatal screening for prevalence of hearing impairment in rural areas. Indian J Otolaryngol Head Neck Surg 70(3):380–386
Paul AK (2011) Early identification of hearing loss and centralized newborn hearing screening facility-the Cochin experience. Indian Pediatr 48(5):355–359
Rehabilitation Council of India, “Status of disability in India-2000,” New Delhi, 2000, pp. 172–185.
Jewel J, Varghese P, Singh T et al (2013) Newborn hearing screening—experience at a tertiary hospital in northwest India. Int J Otolaryngol Head Neck Surg 2(5):211–214
Vignesh SS, Jaya V, Sasireka BI, Sarathy K, Vanthana M (2015) Prevalence and referral rates in neonatal hearing screening program using two step hearing screening protocol in Chennai - a prospective study. Int J Pediatr Otorhinolaryngol 79(10):1745–1747
Sharma Y, Mishra G, Bhatt SH, Nimbalkar S (2015) Neonatal Hearing Screening Programme (NHSP): at a rural based tertiary care centre. Indian J Otolaryngol Head Neck Surg 67(4):388–393
Vaid N, Shanbhag J, Nikam R, Biswas A (2009) Neonatal hearing screening - the Indian experience. Cochlear Implants Int 10(Suppl 1):111–114
Satish HS, Anil Kumar R, Viswanatha B (2019) Screening of newborn hearing at a tertiary care hospital in South India. Indian J Otolaryngol Head Neck Surg 71:1383–1390
Verma RR, Konkimalla A, Thakar A, Sikka K, Singh AC, Khanna T (2021) Prevalence of hearing loss in India. Natl Med J India 34(4):216–222
Verkleij ML, Heijnsdijk EAM, Bussé AML, Carr G, Goedegebure A, Mackey AR, Qirjazi B, Uhlén IM, Sloot F, Hoeve HLJ, de Koning HJ (2021) Country-Committees Joint-Partnership of EUSCREEN Study Consortium. Cost-effectiveness of neonatal hearing screening programs: a micro-simulation modeling analysis. Ear Hear 42:909–916
White KR, Vohr BR, Behrens TR (1993) Universal newborn hearing screening using transient evoked otoacoustic emissions: results of the Rhode Island HearingAssessment Project. Seminars Hearing 14:18–29
White KR, Maxon AB (1995) Universal screening for infant hearing impairment: simple, beneficial, and presently justified. Int J Pediatr Otorhinolaryngol 32(3):201–211
Yoshinaga-Itano C, Sedey AL, Coulter DK, Mehl AL (1998) Language of early- and later-identified children with hearing loss. Pediatrics 102(5):1161–1171
World Health Organization. State of hearing and ear care. WHO Regional Office for South-East Asia; 2005.
Year 2019 position statement: principles and guidelines for early hearing detection and intervention programs (2019). Journal of Early Hearing Detection and Intervention, 4(2), 1-44.
Dhawan R, Mathur NN (2007) Comparative evaluation of transient evoked oto-acoustic emissions and brainstem evoked response audiometry as screening modality for hearing impairment in neonates. Indian J Otolaryngol Head Neck Surg 59(1):15–18
Hrncic N, Goga A, Hrncic S, Hatibovic H, Hodzic D (2021) Factors affecting neonatal hearing screening follow-up in developing countries: one institution prospective pilot study. Medeni Med J 36:14–22
Imam SS, El-Farrash RA, Taha HM, Bishoy HE (2013) Targeted versus universal neonatal hearing screening in a single Egyptian centre. ISRN Pediatrics 2013(574937):1–6
Isaacson G (2000) Universal newborn hearing screening in an inner-city, managed care environment. Laryngoscope 110(6):881–894
Acknowledgements
All staff of Civil Hospital Panchkula.
Funding
None.
Author information
Authors and Affiliations
Contributions
VR, RA, JS, and AG were all involved in conceptualization, drafting, and carrying out the study. All authors were equally involved in drafting ang editing the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Study was ethically approved by Institutional Ethics Committee: Biomedical Research Ethics Committee, Pt. B.D. Sharma Post Graduate Institute of Medical Sciences, Rohtak. Ethical Approval Number: BREC/20/217. Written informed consent was obtained from the mothers/parents/guardians.
Consent for publication
Written and verbal consent obtained from parents/guardians of neonates.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rawat, V., Arora, R., Singh, J. et al. Incidence of hearing loss in neonates at a secondary care hospital in North India—a pilot UNHS study. Egypt J Otolaryngol 39, 120 (2023). https://doi.org/10.1186/s43163-023-00482-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43163-023-00482-4