Abstract
Background
Infections in neonates are mainly caused by sepsis and are the most important complications of low birth weight. In the treatment of these infections, it is common to use broad-spectrum antibiotics such as cholestin. Cholestin can cause changes in the latency of brainstem-evoked response waves. The current cohort study tried to investigate the probability of changes caused by cholestin in LBW neonates and benefiting from two common tests to identify the hearing system in neonates after treatment by cholestin and compared them with a control group.
Methods
The study was an exposure-based cohort, during which hearing damage caused by receiving cholestin was evaluated in 104 low birth weight neonates, in two groups (52 exposure and 52 no-exposure to cholestin). OAE and ABR tests were performed at the age of 3 months to identify complications in two groups and their results were compared.
Results
The absolute latency of waves I, III, and V in the brainstem evoked response test with an intensity of 80 dB Hl showed a significant difference in the exposure group with the control group. The interpeak latency of the waves as well as the effect of the drug on the gender of the infants did not show significant differences with the control group.
Conclusion
Cholestin did not affect the axonal or synaptic transmission time of the auditory nerve to the brainstem. It is possible that the simultaneous presence of risk factors, such as the use of cholestin, low birth weight, noises in the intensive care unit, and other unknown factors, can be effective in the change of the absolute latency of waves.
Similar content being viewed by others
Background
Drug ototoxicity is one of the major preventable side effects of antibiotics [1]. Antibiotic drugs are used in neonates for sepsis, gram-negative infections, and fibrotic cysts. They can cause 2 to 20% hearing damage and affect cochlear and vestibular cells. These drugs’ main effect is on hair cells and protective structure, as well as auditory nerve cells. Their negative effect can vary depending on the patient’s age, antibiotic dosage, simultaneous use with other drugs, and environmental noise [2, 3]. Ototoxicity can occur at any age and affect the hearing and balance system to different degrees. The effect of different drugs on the hearing system has been investigated. Monitoring and impact monitoring protocols are also available, especially in children [4]. However, due to the incomplete hearing system, infants and especially premature neonates may be in more danger of drug side effects and ototoxicity [5].
Low birth weight (LBW) is defined as a birth weight below 2500 g, and it is a common problem in pediatrics, especially in developing countries [6]. The lower the weight of neonates at birth, the higher the probability of their death [7]. The rate of low birth weight deliveries has decreased with the increase in health measures, but this group of babies is still considered one of the most important groups in pediatrics due to the high risk of mortality and morbidity in them. They are also more likely to have a low Apgar score and need to be hospitalized in the neonatal intensive unit (NICU) [8, 9]. Neonatal infections, which mainly cause sepsis and meningitis, are the most important complications of low birth weight and are estimated to be the cause of 1.6 million deaths per year in developing countries [10].
Diagnosing and treating neonatal infections is very important in the reduction of their morbidity and mortality. The treatment usually consists of empirical antibiotic therapy [11]. Nosocomial infections caused by multidrug-resistant gram-negative bacteria have led to the re-use of the injectable form of cholestin, which was used in the past decades. Cholestin or polymyxin E is a cationic lipopeptide antibiotic. Cholestin is administered intravenously and contains colistimethate sodium (CMS) as an inactive prodrug. This drug has been available for more than 60 years, but it was not used for many years due to concerns about nephrotoxicity and the availability of other antibiotics with less toxicity [12]. Currently, cholestin is used as a last choice for the treatment of gram-negative infections that are resistant to several antibiotics, especially in critically ill patients. It is reported that cholestin is effective in more than 70% of cases of infection in LBW neonates [13, 14]. Few studies have been conducted on the safety and efficacy of the injectable form of cholestin in term and pre-term neonates [14]. Possible important side effects of cholestin, in order of incidence, include renal complications, seizures, and apnea, which can have long-term consequences [12,13,14,15].
The interaction of polymyxin with neurons that have high lipids leads to the occurrence of neurological complications which is dose-dependent neurological toxicities reported include confusion, generalized weakness or non-muscular weakness, peripheral and facial paresthesia, relative deafness, visual impairment, dizziness, confusion, seizures, ataxia, neuromuscular block, and pseudo-myasthenic syndrome [16]. One of the important side effects of cholestin is its ototoxicity [17, 18]. LBW neonates who are under treatment with cholestin may suffer from other problems caused by premature birth [18]. Also, there is little evidence regarding the effects of this drug on the auditory system of LBW neonates [18].
The effects of ototoxic drugs, including cholestin, on the auditory system, are usually investigated with the help of auditory brainstem responses (ABR) and otoacoustic emission (OAEs) [19]. Cholestin can reduce the Transient Evoked Otoacoustic Emission (TEOAE) and Distortion product otoacoustic emissions (DPOAE) response by affecting the hair and cochlear cells, as well as increasing the latency and decreasing the amplitude of auditory brainstem responses (ABR) [19].
The auditory brainstem-evoked response is a non-invasive electrophysiological method that is being used to investigate the maturation and firing synchrony of neurons in the auditory nerve pathways. The three main waves in this test, and their depth and amplitude show the growth and synchrony of a set of auditory nerve fibers. Wave I is the result of the function of the distal part of the auditory nerve, and its recording indicates the effective activity of auditory nerve afferents leaving the cochlea and entering the internal auditory canal. Wave III is the result of the function of the neurons of the second level of hearing in the cochlear nuclei and is considered the same source of stimulation. Wave V is the result of the activity of neurons in the lateral lemniscus entering the inferior colliculus, which is the result of the activity of the opposite or contralateral pathway to stimulation [5, 20]. The absolute latency of each wave is from the time of sound stimulus to the simultaneous response time of a set of auditory nerve fibers. The distance between these waves is a relative criterion that examines the general indicator of the transmission time of the auditory nerve path to the brain stem [21]. Especially, the latency between the peaks of the waves indicates the axonal transmission time along the nerve and the synaptic latency between neurons [20].
Some studies showed that cholestin can cause permanent hearing loss, especially in high frequencies [4, 18]. The latency of ABR waves can be affected by factors such as age and immaturity of the auditory pathway, use of ototoxic drugs, and peripheral or central pathological and non-pathological factors [21]. The changes in the latency of wave I are more affected by environmental factors and the changes in the latency of other waves can be a sign of other damage to the central auditory pathway [20]. While, in the literature, cholestin has been introduced as an ototoxic drug, and little information is available about its negative effects on hearing with physiological and electrophysiological tests [4]. Hearing impairment can cause many social, emotional, and academic problems in children. Early diagnosis and treatment of hearing impairment improve the communication development of these neonates. The current cohort study tried to investigate the probability of harm caused by cholestin in LBW neonates and benefiting from two common tests to identify hearing damage in neonates after treatment by cholestin and compared them with a control group. The current cohort study tried to investigate the probability of changes caused by cholestin in LBW neonates and benefiting from two common tests to identify the hearing system in neonates after treatment by cholestin and compared them with a control group.
Methods
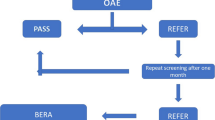
The study was an exposure-based cohort, during which hearing damage caused by receiving cholestin was evaluated in 104 low birth weight neonates, in two groups (52 exposure and 52 no-exposure to cholestin). The study sample was chosen by a convenience sampling method from premature neonates weighing less than 2500 g who were admitted to the neonatal intensive care unit of Shahid Akbarabadi and Ali Asghar Hospital in Tehran from 2018 to 2019. Inclusion criteria were gestational age of fewer than 37 weeks, low birth weight (less than 2500 g), hospitalization in the NICU, parental consent for the neonate to participate in the study, and the absence of congenital anomalies and pass in Otoacoustic emission test (OAE) and ABR hearing screening test. Cholestin and other antibiotics were prescribed during the disease and based on the needs of the neonates, and no intervention was performed in the two groups.
The exposure group was neonates who were treated with cholestin during the disease due to treatment-resistant clinical sepsis or positive blood culture. The second group was the non-exposure group that did not need to receive cholestin. The non-exposure group was selected from the hospitalized neonates of the same NICUs with the same inclusion criteria as the exposure group. The two groups were matched in terms of gender, birth weight, gestational age, duration of NICU hospitalization, medications, mechanical ventilation duration, delivery method, and family history of hearing damage. Diagnostic OAE and ABR tests were performed at the age of 3 months to identify complications in two groups, and their results were compared. In cases where the hearing loss of a newborn required further follow-up, the necessary information was given to the families, and they were referred to specialized centers.
Recording the evoked responses of the baby were done in sleep mode and a quiet environment. No sedatives were used and all infants were evaluated in normal sleep after feeding. All infants were examined by using the ICS EP charter 200 system (Otometrics, Co. Denmark) for auditory evoked brainstem response (ABR) with click stimulus. The components examined in the ABR test included latency of waves I, III, and V, interpeak intervals of I–III, III–V, and I–V, as well as the threshold of wave V. Stimulus intensity was started at 80 dB NHL (normal hearing level) and decreased to near-threshold levels. We used the inserted phone as a single phone with 2000 sweeps and the stimulus rate was 27.3C⁄S with a bandpass filter of 100–3000 Hz. The time window was set at 15 ms with a pre-stimulation time of -1 ms and 100,000 times amplification, and ABR waves were recorded through electrodes on the high forehead (non-inverting) and bilateral mastoid for ground and inverting [20]. The DPOAE test was performed by using the Capella2 Madsen system. The presence of OAE was defined by SNR of ≥ 6. Also, we replicated runs to find reliable amplitudes [20].
Statistical analysis
The data were analyzed by SPSS version 22. The normality of the distribution of continuous variables was checked by skewness and kurtosis. If the skewness was between -1.5 and 1.5 and the skewness was between 4.5 and 1.5, the distribution of the variable was considered normal. Continuous variables were reported as mean (standard deviation) or median (interquartile range) and nominal variables using frequency (percentage). Depending on the distribution of continuous variables, the t test or the non-parametric mean comparison test was used to compare continuous variables between the two groups. The chi-square test was used to compare nominal variables between groups.
Ethical consideration
The study protocol was approved by the Biomedical Research Ethics Committee of the Iran University of Medical Sciences (IR.IUMS.FMD.REC.1399.003). The project was conducted under the ethical principles and the national norms and standards for conducting Medical Research in Iran. The aim of the study and the methodology was explained to the parents of neonates and informed written consent was received from the. All cases with hearing disorders were referred to a specialist. No fees for diagnostic tests were received from the research units.
Results
At the end of the study, 52 neonates were examined in each group, and no cases were lost to follow-up. The median (interquartile range) of Apgar scores 1 and 5 min were 6 (5–7) and 8 (7–9), respectively, and were the same in both groups. Based on the comparison of the two groups in Table 1, there was no significant difference between the characteristics of the neonates in the two groups.
To compare response variables between cholestin and control groups, generalized estimating equation (GEE) models were fitted, in which the effect of binaural measurements was controlled. The findings are reported in the form of the mean (standard deviation) in Table 2.
The comparison of the interpeak latency of waves and the absolute latency of waves showed that there were significant differences between the absolute latency of all waves. Also, the interpeak latency of waves was not significantly different.
According to Table 2, the absolute latency of waves I, III, and V in the ABR test was significantly lower in the control group than in the cholestin group, but in the case of interpeak latency (I–III, III–V, and I–V), no significant relationship was found between the two groups.
Discussion
In the present study, we investigated the possible effects of cholestin on the hearing system of neonates who have several risk factors for hearing loss. Ototoxic drugs have different degrees of cochleotopic and vestibulotoxic effects, which can cause damage in different age groups [22, 23]. Therefore, it is recommended to have constant monitoring during their use [22, 23]. The most common side effects of cholestin are neurotoxicity and nephrotoxicity, which are mostly manifested as visual problems and neuromuscular developmental problems, dizziness, and ataxia (15). The path of the auditory nerve to its final processing center in the auditory cortex is a complicated path and several factors can affect the direction of the sound message. The biggest concern is the negative effects of drugs on the age group of children and babies, where the delay in identifying hearing problems in them can be associated with the delay in the development of speech and language and subsequently the social and educational development.
The present study, in line with previous studies that investigated the effects of cholestin and ototoxic drugs, did not find a significant effect on interpeak latency and nerve conduction or synaptic latency [2, 4]. In a study conducted by Sarıca et al. in 2018, there was only one case of an increase in latency at the level of 15 dB and equivalent to the threshold, which can be a common increase in this intensity range. However, it should be analyzed according to the conditions and the device used [4]. In the present study, no difference was observed in tracking the hearing threshold of newborns between the control group and the group using cholestin. Decreasing the intensity of the stimulus, up to the approximate threshold of hearing, the V wave showed the possibility of reliability and reproducibility in both investigated groups. In other words, no significant difference was seen between the two groups, which indicates that the firing of nerve fibers did not occur at low intensity and the drug cholestin did not have a significant effect on the firing time of nerve fibers [4, 20]. In addition, there was no significant difference between the control group and the drug-receiving group in the comparison of the signal-to-noise ratio in the ear acoustic emissions (OAE) test, where a criterion higher than 6 dB was considered the norm. Acceptable responses were observed in both groups, which indicated the health of cochlear cells (outer hair cells). OAEs and ABR are the gold standards for diagnosing cochlear hair damage in ototoxicity. Before any obvious damage in the behavioral test, the damage of sensory hairs can indicate the occurrence of ototoxicity. In the present study, before diagnostic tests, all infants were screened by the OAE method, and if there was a response, they were included in the study. Therefore, observing the reliable ranges of ear emissions indicates the health of cochlear sensory hairs [24]. The result of this study can be considered in line with the results of the study by Hoog et al. in 2003, in which only in one case the response of acoustic emissions was removed. It seems that the elimination of ear emissions in such cases is due to the presence of vernix and the remains of childbirth during the examination of the newborn, which can be achieved with the help of high-frequency tympanometry to differentiate these two disorders [2].
The important result of the current study is the increase in the absolute latency of the three main waves I, III, and V without increasing the interpeak latencies. The possible explanation is that since the hearing thresholds of neonates were examined up to the norm, and in all cases, wave V was detected in the range of 15 to 20 dB Hl. In addition, the responses of emissions were within the normal range. Therefore, the possibility of sensory or cochlear loss that can cause the increase in the latency in frequencies from 1000 to 4000 Hz is low. Also, there is no possibility of damage or conductive loss that may cause an increase in the same latency in the main and interpeak waves [20]. This increase in latency is not the result of cholestin, and it does not increase the absolute latency of waves, but other risk factors that have led to the hospitalization of the neonate in the intensive care unit, such as anemia or infections can lead to an increase in the latency of wave I [5]. In addition, in neonates, increases in latency may be due to minor damage in the auditory nerve conduction path, the cause of which is completely unclear. Moreover, 2 to 15% of cases of hearing loss in premature neonates are from unknown causes [25]. The evidence indicates that various factors such as the combination of environmental, genetic, and pharmaceutical factors during hospitalization in the intensive care unit and the presence of noise in this environment can affect the developmental status of the auditory system in neonates [3].
While there have been studies on the effects of high-dose levels of drugs in the blood serum on the auditory system, it is still difficult to distinguish their effects from other confounding factors, especially in infancy. In this study, as in other studies, it was not possible to separate the risk factors and the probability of hearing damage for each neonate [3, 21], and it was only possible to compare these factors with the control group. Children who passed the hearing screening test were included in the study. Unlike previous studies, instead of using the screening approach [2], the path of the cochlea to the brainstem was investigated. These two factors strengthen the result of the study as only neonates with healthy auditory systems entered the study, and they were assessed with strong and available methods.
Conclusion
Based on the results of the present study, it can be concluded that cholestin does not affect the pathway of nerve conduction through axons and synapses in auditory neurons. The observed increases in the absolute latency of waves in this study are better to be investigated further in more controlled conditions where the risk and influencing factors are controllable and also with a larger sample size. We suggest checking the effect of cholestin on low birth weight and premature neonates. Diagnostic ABR should be done before the start of cholestin and 72 h after the start of cholestin and then weekly during the treatment and after the end of the treatment with regular intervals.
Limitations
The most important limitation of the present study was that in this study, same as similar studies, it was not possible to separate the factors affecting the auditory system from the effect of the drug, due to the numerous factors affecting it and the special conditions of the neonates.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not available but they can be asked from the corresponding author at reasonable request.
References
Guo J, Chai R, Li H, Sun S (2019) Protection of Hair Cells from Ototoxic Drug-Induced Hearing Loss. In: Li H, Chai R (eds). Hearing Loss: Mechanisms, Prevention and Cure. Advances in Experimental Medicine and Biology, vol 1130. Springer, Singapore. https://doi.org/10.1007/978-981-13-6123-4_2
de Hoog M, van Zanten BA, Hop WC, Overbosch E, Weisglas-Kuperus N, van den Anker JN (2003) Newborn hearing screening: tobramycin and vancomycin are not risk factors for hearing loss. J Pediatr 142(1):41–6. https://doi.org/10.1067/mpd.2003.mpd037 Epub 2003/01/10 PubMed PMID: 12520253
Lord SG (2019) Monitoring protocols for cochlear toxicity. Semin Hear 40(2):122–43. https://doi.org/10.1055/s-0039-1684042 Epub 2019/05/01 PubMed PMID: 31036990; PubMed Central PMCID: PMCPMC6486360
Sarica S, Yurttutan S (2018) An evaluation of hearing in infants administered with colistin in the premature neonatal intensive care unit. J Matern Fetal Neonatal Med 31(21):2918–22. https://doi.org/10.1080/14767058.2018.1479388 Epub 2018/05/22 PubMed PMID: 29779419
Amin SB, Orlando M, Eddins A, MacDonald M, Monczynski C, Wang H (2010) In utero iron status and auditory neural maturation in premature infants as evaluated by auditory brainstem response. J Pediatr 156(3):377–81. https://doi.org/10.1016/j.jpeds.2009.09.049 Epub 2009/11/27 PubMed PMID: 19939407; PubMed Central PMCID: PMCPMC2827634
Talie A, Tessema M, Alemayehu M (2019) Magnitude of Low Birth Weight and Associated Factors among Newborns Delivered in Dangla Primary Hospital, Amhara Regional State, Northwest Ethiopia, 2017. J Pregnancy 2019:1–6. https://doi.org/10.1155/2019/3587239
Lequien P, Zaoui C, Lecoutour X, Dubos JP, Duquennoy C, Bouchez MC et al (1989) Contribution to the definition of high-risk groups of low birth weight infants. Rev Epidemiol Sante Publique 37(2):119–125 Epub 1989/01/01 PubMed PMID: 2772356
Arias F, Tomich P (1982) Etiology and outcome of low birth weight and preterm infants. Obstet Gynecol 60(3):277–281 Epub 1982/09/01 PubMed PMID: 7121906
Mittendorf R, Herschel M, Williams MA, Hibbard JU, Moawad AH, Lee KS (1994) Reducing the frequency of low birth weight in the United States. Obstet Gynecol 83(6):1056–9. https://doi.org/10.1097/00006250-199406000-00031 Epub 1994/06/01 PubMed PMID: 8190423
Vergnano S, Sharland M, Kazembe P, Mwansambo C, Heath PT (2005) Neonatal sepsis: an international perspective. Arch Dis Child Fetal Neonatal Ed 90(3):F220-4. https://doi.org/10.1136/adc.2002.022863 Epub 2005/04/23 PubMed PMID: 15846011; PubMed Central PMCID: PMCPMC1721871
Polin RA, Committee on F, Newborn (2012) Management of neonates with suspected or proven early-onset bacterial sepsis. Pediatrics 129(5):1006–15. https://doi.org/10.1542/peds.2012-0541 Epub 2012/05/02 PubMed PMID: 22547779
Nation RL, Li J (2009) Colistin in the 21st century. Curr Opin Infect Dis 22(6):535–43. https://doi.org/10.1097/QCO.0b013e328332e672 Epub 2009/10/03 PubMed PMID: 19797945; PubMed Central PMCID: PMCPMC2869076
Cagan E, Kiray Bas E, Asker HS (2017) Use of colistin in a neonatal intensive care unit: a cohort study of 65 patients. Med Sci Monit 23:548–54. https://doi.org/10.12659/msm.898213 Epub 2017/01/31 PubMed PMID: 28135233; PubMed Central PMCID: PMCPMC5295175 no conflicts of interest to report
Jajoo M, Kumar V, Jain M, Kumari S, Manchanda V (2011) Intravenous colistin administration in neonates. Pediatr Infect Dis J 30(3):218–21. https://doi.org/10.1097/INF.0b013e3182064bfe Epub 2011/01/20 PubMed PMID: 21245777
Lulic-Botica M, Dejesus L (2011) Colistin therapy in a 23-week gestational-age neonate with multidrug-resistant Acinetobacter baumannii pneumonia. AJP Rep 1(1):7–10. https://doi.org/10.1055/s-0030-1271217 Epub 2011/09/01 PubMed PMID: 23705076; PubMed Central PMCID: PMCPMC3653549
Falagas ME, Kasiakou SK (2006) Toxicity of polymyxins: a systematic review of the evidence from old and recent studies. Crit Care 10(1):R27. https://doi.org/10.1186/cc3995 Epub 2006/03/02 PubMed PMID: 16507149; PubMed Central PMCID: PMCPMC1550802
Vairo C, Vidal MV, Hernandez RM, Igartua M, Villullas S (2023) Colistin-and Amikacin-loaded lipid-based drug delivery systems for resistant gram-negative lung and wound bacterial infections. Int J Pharm 635:122739
Yurttutan S, Atalay BC (2021) Short and long term side effect of colistin treatment in preterm infants. Turk J Pediatr 63(6):1108–1109
Suwannatrai P, Chaiyakulsil C (2022) Hearing screening outcomes in pediatric critical care survivors: a 1-year report. Acute Crit Care 37(2):209–216
Hall JW (2007) New handbook of auditory evoked responses. Pearson, Boston
da Silva D, Lopez P, Mantovani JC (2015) Auditory brainstem response in term and preterm infants with neonatal complications: the importance of the sequential evaluation. Int Arch Otorhinolaryngol 19(02):161–165
Ramma L, Schellack N, Heinze B (2019) Prevention of treatment-induced ototoxicity: an update for clinicians. S Afr Med J 109(3):145–149
Rizk HG, Lee JA, Liu YF, Endriukaitis L, Isaac JL, Bullington WM (2020) Drug-induced ototoxicity: a comprehensive review and reference guide. Pharmacotherapy 40(12):1265–75
Hall JW, Swanepoel DW (2010) Objective assessment of hearing. Plural, San Diego, Abingdon
Ganesan P, Schmiedge J, Manchaiah V, Swapna S, Dhandayutham S, Kothandaraman PP (2018) Ototoxicity: a challenge in diagnosis and treatment. J Audiol Otol 22(2):59–68. https://doi.org/10.7874/jao.2017.00360 Epub 2018/02/24 PubMed PMID: 29471610; PubMed Central PMCID: PMCPMC5894487
Acknowledgements
The authors would like to express their deepest gratitude to Iran university of Medical Sciences.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
N. Kh. and M. A. conceived of and designed the study. M. M., M. K., N. K., and M. A. conducted the interviews. Z. Sh. and S. A. analyzed the data. N. Kh. and M.A. wrote the manuscript draft. All authors contributed to reviewing and editing the manuscript. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the Biomedical Research Ethics Committee of the Iran University of Medical Sciences (IR.IUMS.FMD.REC.1399.003). The project was conducted under the ethical principles and the national norms and standards for conducting Medical Research in Iran. The aim of the study and the methodology was explained to the parents of neonates, and informed written consent was received from the. All cases with hearing disorders were referred to a specialist. No fees for diagnostic tests were received from the research units.
Consent for publication
N/A.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khosravi, N., Mazaheryazdi, M., Kalani, M. et al. Physiological and electrophysiological evaluation of the hearing system in low birth weight neonates treated with cholestin: a cohort study. Egypt J Otolaryngol 39, 75 (2023). https://doi.org/10.1186/s43163-023-00425-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43163-023-00425-z