Abstract
Background
The aim of our study was to assess whether there is role of obesity in ENT diseases like otitis media effusion, chronic otitis media, chronic rhinosinusitis, sudden sensorineural hearing loss and chronic tonsillitis, or not.
Methods
The present prospective study, after approval by institutional ethics committee, was conducted in the Department of ENT, SMGS Hospital, GMC Jammu from January 2021 to February 2022 on 590 patients, who were divided into 6 groups — group A — otitis media with effusion (n = 95 patients), group B — chronic otitis media (n = 171 patients), group C — sudden SNHL (n = 43 patients), group D — chronic rhinosinusitis (n = 102 patients), group E — chronic tonsillitis (n = 67 patients) and group F (control group) — patients (aged 11–50 years) coming to ENT OPD with other problems, except those problems mentioned in inclusion and exclusion criteria (n = 112 patients). Severity of disease was evaluated using Adelaide Disease Severity Score (CRS patients), otoscopy and pure tone audiometry (OME and COM), pure-tone audiometry (sudden SNHL) and Brodsky grading scale (chronic tonsillitis). Mean BMI and percentage of obese patients were calculated for each group.
Results
The mean age of presentation in our study was 40.66 ± 7.25 years. Male to female ratio was 1:1.6 in our study. The mean BMI in control group (group F) was 22.51 ± 3.01 kg/m2. The mean BMI was 25.41 ± 2.81 kg/m2 in group A, 25.33 ± 2.34 kg/m2 in group B, 25.12 ± 3.14 kg/m2 in group C, 25.78 ± 2.33 kg/m2 in group D and 25.03 ± 1.84 kg/m2 in group E, the difference between each of these groups and control group being statistically significant (p < 0.005). The percentage of obese patients in group F was 20.5% (23 patients). The percentage of obese patients was 53.6% (51 patients) in group A, 49.7% (85 patients) in group B, 39.5% (17 patients) in group C, 54.9% (56 patients) in group D and 31.3% (21 patients) in group E. Upon comparison with group F, the difference in percentage of obese patients was statistically significant in each group. Obese patients were more likely to have otitis media with effusion (OR 1.85, 95% CI 0.15 to 6.49), chronic otitis media (OR 1.80, 95% CI 0.15 to 6.33), sudden SNHL (OR 1.62, 95% CI 0.21 to 6.40), chronic rhinosinusitis (OR 2.05, 95% CI 0.15 to 6.55) and chronic tonsillitis (OR 1.60, 95% CI 0.16–6.13), than the control group.
Conclusion
Obesity leads to various ENT problems by altering the immune system. In our study, mean BMI was significantly higher in patients with otitis media effusion, chronic otitis media, chronic rhinosinusitis, sudden sensorineural hearing loss and chronic tonsillitis and also, as the severity of disease increased with increase in severity of BMI, showing positive correlation for all study groups, thus establishing association of obesity and these common otorhinolaryngological conditions.
Similar content being viewed by others
Background
Obesity is officially considered as a disease by WHO and various national/international organisations [1]. Around 650 million people are categorised as obese globally. In India, there are about 135 million obese people. The current trajectory of prevalence acceleration would result in about 50% of world’s population being obese by 2030 [2].
Obesity is the accumulation of abnormal or excessive fat that may interfere with the maintenance of an optimal state of health. Obesity is recognised as a rapidly increasing major health problem globally, with people having body mass index (BMI = weight divided by square of height) value greater than 25 kg/m2 considered as obese. Body mass index (BMI) is the most widely used representative measure of obesity and is well-correlated with chronic diseases and mortality risk [3].
Obesity is basically a systemic chronic inflammatory disease, leading to musculoskeletal overload and chronic diseases such as diabetes, heart diseases, renal diseases and cerebrovascular disease [4]. Obesity is associated with increased number of macrophages in adipose tissue, which stimulates higher release of inflammatory mediators such as tumour necrosis factor alpha, interleukin-6, C-reactive protein and adiponectin, as compared to underweight or normal weight individuals. This leads to generation of a pro-inflammatory state and oxidative stress in the human body [5].
Increased adipose tissue located in upper airway and head-neck region acts as an endocrine organ and modifies immunity, thus leading to various otorhinolaryngological conditions such as otitis media (otitis media with effusion, chronic otitis media, acute otitis media, recurrent otitis media), hearing loss (sudden sensorineural hearing loss, age-related hearing loss, noise induced hearing loss), chronic rhinosinusitis, obstructive sleep apnoea, laryngopharyngeal reflux and head neck cancers [6].
Also, there is chronic sympathetic overactivity in obese individuals, leading to dysregulation of immune system, induction of a chronic inflammatory state and increased insulin resistance. All these pathological mechanisms further pave way for occurrence of various otorhinolaryngological diseases [7].
Despite many research studies on association of obesity-induced systemic inflammation with various chronic diseases affecting human body like type 2 diabetes mellitus and cardiovascular diseases, very less literature is present that shows any association of obesity with otorhinolaryngological inflammatory conditions other than obstructive sleep apnoea, especially in our local population. In this study, we aim to assess whether there is role of obesity in ENT diseases like otitis media effusion, chronic otitis media, chronic rhinosinusitis, sudden sensorineural hearing loss and chronic tonsillitis, or not.
Methods
The present prospective study, after approval by institutional ethics committee, was conducted in the Department of ENT, SMGS Hospital, GMC Jammu from January 2021 to February 2022.
Inclusion criteria
-
1)
Age: 11 to 50 years
-
2)
Otitis media with effusion, diagnosed as per otoscopy and impedance audiometry
-
3)
Chronic otitis media, diagnosed as per otoscopy and CT scan temporal bone
-
4)
Sudden SNHL, diagnosed as per pure tone audiometry (reduction in hearing threshold of 30 dB or more, over 3 days in three consecutive frequencies)
-
5)
Chronic rhinosinusitis, diagnosed as per CT scan of nose and paranasal sinuses
-
6)
Chronic tonsillitis, diagnosed clinically and confirming history of recurrent sore throat/fever
Exclusion criteria
-
1)
Age less than 11 years and more than 50 years
-
2)
The presence of systemic diseases
-
3)
Head-neck malignancies
-
4)
Congenital or acquired immunodeficiencies
-
5)
Other predisposing factors for these ENT conditions like habitual factors (smoking, alcoholism, lifestyle, sporting habitus), environmental factors, occupational factors (noise exposure, exposure to allergens), sanitation/hygiene factor, local anatomical variations (deviated nasal septum) and history of atopy
A total of 590 patients were included in our study after informed written consent. Relevant clinical history was taken, and general/systemic/local ENT examination was done. Routine blood investigations (CBC, RFT, LFT, lipid profile and coagulation profile), relevant radiological and relevant audiological investigations were done.
All subjects were divided into 6 groups:
-
▪ Group A — Otitis media with effusion (OME) (n = 95 patients)
-
▪ Group B — Chronic otitis media (COM) (n = 171 patients)
-
▪ Group C — Sudden sensorineural hearing loss (sudden SNHL) (n = 43 patients)
-
▪ Group D — Chronic rhinosinusitis (CRS) (n = 102 patients)
-
▪ Group E — Chronic tonsillitis (n = 67 patients)
Group F (control group) — These are patients (aged 11–50 years) coming to ENT OPD with other problems, except those problems mentioned in inclusion and exclusion criteria (n = 112 patients).
BMI (body mass index) was calculated for all patients in each group, using the following formula:
Mean BMI was calculated in each of the six groups.
Also, total number of obese patients was calculated for each group based on BMI, using standards of WHO for Asia–Pacific region (Table 1).
Severity of disease was evaluated using Adelaide Disease Severity Score (CRS patients), otoscopy and pure-tone audiometry (OME and COM), pure-tone audiometry (sudden SNHL) and Brodsky Grading scale (chronic tonsillitis).
Adelaide Disease Severity Score [8]: It is simplified tool to assess CRS symptoms and quality of life. Individual symptom scores were added to a total out of 25. A VAS was completed by the subject so as to indicate their quality of life on a scale ranging from 0 to 7, O showing no effect and 7 indicating maximal effect (Table 2). The combined symptom score and VAS score were added to a total score out of 32.
Brodsky tonsil size grading (Table 3) [9]
All data was entered in Microsoft Excel spreadsheet and analysed and compared using the Statistical Package for Social Sciences (SPSS) software (version 21 for windows). Appropriate statistical analytical tests were applied as per the advice of statistician.
Results
A total of 590 patients were included in our study. The mean age of presentation in our study was 40.66 ± 7.25 years. The mean age of presentation was 39.16 ± 6.55 years in group A, 41.22 ± 5.32 years in group B, 33.12 ± 8.17 years in group C, 44.19 ± 7.15 years in group D, 35.26 ± 5.33 years in group E and 37.11 ± 4.19 years in group F, there being no statistically significant difference between each of the experimental group (groups A, B, C, D, E) and control group (group F) being statistically insignificant (p-value = 0.65).
Male to female ratio was 1:1.6 in our study — M:F ratio being 1:1.7 in group A, 1:1.3 in group B, 1:1.3 in group C, 1:1.1 in group D, 1:1.6 in group E and 1:1.1 in group F. There was female preponderance in all groups, with the difference between each of the experimental group (groups A, B, C, D, E) and control group (group F) being statistically insignificant (p-value = 0.34).
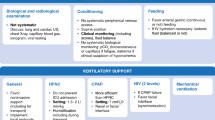
The percentage of obese patients (Fig. 1) in group F was 20.5% (23 patients). The percentage of obese patients was 53.6% (51 patients) in group A, 49.7% (85 patients) in group B, 39.5% (17 patients) in group C, 54.9% (56 patients) in group D and 31.3% (21 patients) in group E. Upon comparison with group F, the difference in percentage of obese patients was statistically significant in each of the experimental group (group A: p = 0.0001, group B: p = 0.0001, group C: p = 0.0029, group D: p = 0.001 and group E: p = 0.034). Odds ratio — OR (adjusted for age, sex and local/general predisposing factors) for each case group when compared to control group was calculated — obese patients were more likely to have otitis media with effusion (OR 1.85, 95% CI 0.15 to 6.49), chronic otitis media (OR 1.80, 95% CI 0.15 to 6.33), sudden SNHL (OR 1.62, 95% CI 0.21 to 6.40), chronic rhinosinusitis (OR 2.05, 95% CI 0.15 to 6.55) and chronic tonsillitis (OR 1.60, 95% CI 0.16–6.13), than the control group.
As per WHO Asia–Pacific BMI value, in OME group (n = 95), the average hearing impairment in OME group as per BMI was 28.5 dB in obese patients (n = 51), 26 dB in overweight (n = 32), 22.5 dB in normal (n = 8) and 22 db in underweight patients (n = 4), showing positive correlation between severity of OME and severity in grading of BMI (Pearson correlation coefficient = 0.77). In COM group (n = 171), average hearing impairment was 41.5 dB in obese (n = 85), 35 dB in overweight (n = 61), 32.5 dB in normal (n = 11) and 31 dB in underweight (n = 14), showing positive correlation between severity of CRS and BMI (Pearson correlation coefficient = 0.90). In sudden SNHL group (n = 43), average hearing impairment was 44 dB in obese (n = 17), 40 dB in overweight (n = 15), 38.5 dB in normal (n = 6) and 38 dB in underweight (n = 5), showing positive correlation between severity of CRS and BMI (Pearson correlation coefficient = 0.49).
In CRS group (n = 102), mean Adelaide score was 22.31 in obese patients (n = 56), 22.1 in overweight patients (n = 26), 17.5 in normal (n = 14) and 17.2 in underweight (n = 6), showing positive correlation between severity of CRS and BMI (Pearson correlation coefficient = 0.83). In chronic tonsillitis group (n = 67), majority of patients (81.2%) in obese category (n = 21) had grade 4 tonsil size, majority (79.4%) of overweight patients (n = 17) had grade 3 tonsil size, majority of patients (78.1%) having normal BMI (n = 15) had grade 2 tonsil size and majority of patients having (77.9%) underweight BMI (n = 14) had grade 2 tonsil size, showing positive correlation between severity of CRS and BMI (Pearson correlation coefficient = 0.10).
The mean BMI (Table 4) in control group (group F) was 22.51 ± 3.01 kg/m2. The mean BMI was 25.41 ± 2.81 kg/m2 in group A, the difference between groups A and F being statistically significant (p = 0.0001). The mean BMI was 25.33 ± 2.34 kg/m2 in group B, the difference between groups B and F being statistically significant (p = 0.0001). The mean BMI was 25.12 ± 3.14 kg/m2 in group C, the difference between groups C and F being statistically significant (p = 0.0012). The mean BMI was 25.78 ± 2.33 kg/m2 in group D, the difference between groups D and F being statistically significant (p = 0.0001). The mean BMI was 25.03 ± 1.84 kg/m2 in group E, the difference between groups E and F being statistically significant (p = 0.004).
Discussion
Obesity, defined as excess body weight for a given height, is a crucial global problem with life-threatening consequences. The associated excess adiposity evolves slowly, with long-term-positive energy balance. There is apoptosis of adipocytes, leading to tissue remodelling and eventual high number of macrophages — all this leading to a low-grade systemic inflammatory state [10,11,12].
Obese individuals tend to be at higher risk of developing not only diseases like type 2 diabetes mellitus, fatty liver disease and cardiovascular diseases but also various otorhinolaryngological diseases like otitis media with effusion, rhinosinusitis and allergy, laryngopharyngeal reflux disease and post/peri complications of adenostonsillectomy [13].
The mean age of presentation in our study was 40.66 ± 7.25 years, there being no statistically significant difference between each of the experimental group (groups A, B, C, D, E) and control group (group F) being statistically insignificant (p-value = 0.65). This observation was consistent with study conducted by Kim T. H. et al. (2015) [14]. Male to female ratio was 1:1.6 in our study; there was female preponderance in all groups, with the difference between each of the experimental group (groups A, B, C, D, E) and control group (group F) being statistically insignificant (p-value = 0.34). This observation was consistent with study conducted by Nam J. S. et al. (2021) [15]; however, Ahmed S. et al. (2014) [16] showed male preponderance in their study. The difference in gender distribution from previous studies could be due to variation in demographic distribution from one population to other.
The percentage of obese patients in group F was 20.5% (23 patients). The percentage of obese patients was 53.6% (51 patients) in group A, 49.7% (85 patients) in group B, 39.5% (17 patients) in group C, 54.9% (56 patients) in group D and 31.3% (21 patients) in group E. Upon comparison with group F, the difference in percentage of obese patients was statistically significant in each of the experimental group (group A: p = 0.0001, group B: p = 0.0001, group C: p = 0.0029, group D: p = 0.001 and group E: p = 0.034). Obese patients were more likely to have otitis media with effusion (OR 1.85, 95% CI 0.15 to 6.49), chronic otitis media (OR 1.80, 95% CI 0.15 to 6.33), sudden SNHL (OR 1.62, 95% CI 0.21 to 6.40), chronic rhinosinusitis (OR 2.05, 95% CI 0.15 to 6.55) and chronic tonsillitis (OR 1.60, 95% CI 0.16–6.13), than the control group.
Also, in our study, as the severity of disease increased with increase in severity of BMI, showing positive correlation for all study groups. As per WHO Asia–Pacific BMI value, in OME group (n = 95), the average hearing impairment in OME group as per BMI was 28.5 dB in obese patients (n = 51), 26 dB in overweight (n = 32), 22.5 dB in normal (n = 8) and 22 db in underweight patients (n = 4), showing positive correlation between severity of OME and severity in grading of BMI (Pearson correlation coefficient = 0.77). In COM group (n = 171), average hearing impairment was 41.5 dB in obese (n = 85), 35 dB in overweight (n = 61), 32.5 dB in normal (n = 11) and 31 dB in underweight (n = 14), showing positive correlation between severity of CRS and BMI (Pearson correlation coefficient = 0.90). In sudden SNHL group (n = 43), average hearing impairment was 44 dB in obese (n = 17), 40 dB in overweight (n = 15), 38.5 dB in normal (n = 6) and 38 dB in underweight (n = 5), showing positive correlation between severity of CRS and BMI (Pearson correlation coefficient = 0.49).
In CRS group (n = 102), mean Adelaide score was 22.31 in obese patients (n = 56), 22.1 in overweight patients (n = 26), 17.5 in normal (n = 14) and 17.2 in underweight (n = 6), showing positive correlation between severity of CRS and BMI (Pearson correlation coefficient = 0.83). In chronic tonsillitis group (n = 67), majority of patients (81.2%) in obese category (n = 21) had grade 4 tonsil size, majority (79.4%) of overweight patients (n = 17) had grade 3 tonsil size, majority of patients (78.1%) having normal BMI (n = 15) had grade 2 tonsil size and majority of patients having (77.9%) underweight BMI (n = 14) had grade 2 tonsil size, showing positive correlation between severity of CRS and BMI (Pearson correlation coefficient = 0.10).
Now, upon considering each case group individually, we try to explain our study findings. It was found that the mean BMI in group of patients with otitis media with effusion (group A) was 25.44 ± 2.81 kg/m2, which was significantly higher than control group (mean BMI = 22.51 ± 3.01 kg/m2). This was consistent with studies conducted by Kaya S. et al. (2017) [17] and Choi H. G. et al. (2015) [18]. Obesity is characterised by higher concentrations of inflammatory mediators such as IL-6 and TNF alpha, and some studies have shown the presence of both these mediators in middle ear fluid of OME patients [19]. Besides this known association between OME and obesity, we in our study also found that out of 95 patients with OME, 42 patients (44.2%) had associated features suggestive of allergic rhinitis, and 11 patients (11.5%) gave associated history of GERD. Obesity leads to reduced adiponectin levels, which downregulates T cells, leading to altered host immunity and allergy. Allergic rhinitis induces mast cells in nasal mucosa to secrete various inflammatory mediators, resulting in Eustachian tube occlusion and OME [20]. Huang S. L. et al. (1999) [21] also showed in their cross-sectional study that BMI was a significant predictor of allergic rhinitis and OME. However, Sybilski D. et al. (2015) [22] could not find any correlation between obesity and allergic rhinitis. In addition, the reason for GERD in obese individuals of our study could be due to increased intragastric pressure and/or lower oesophageal sphincter incompetence. Thus, obesity led to GERD, which then by way of gastric reflux reaching middle ear through nasopharynx and Eustachian tube resulted in OME [23]. Rodrigues M. M. et al. (2014) [24] also suggested in their study that GERD and obesity are positively correlated.
In our study, the mean BMI was 25.12 ± 2.34 kg/m2 in group B (chronic otitis media), the difference between group B and control group being statistically significant (p = 0.0021). Similar finding was shown by Kim T. H. et al. (2015) [14]. Obesity-associated low-grade inflammation leads to the expression of cytokines (like IL-6, TNF alpha, fibroblast growth factor and bone morphologenetic proteins) involved in the pathogenesis of chronic otitis media, resulting in tissue remodelling and inflammatory cell proliferation [25].
In our study, the mean BMI was 25.78 ± 3.14 kg/m2 in sudden SNHL group (Table 2), the difference of mean BMI between this group and control group being statistically significant (p = 0.0001). Lee J. S. et al. (2015) [26] and Lalwani et al. (2013) [27] also in their study showed that increased BMI is significantly associated with prevalence of sudden SNHL. However, Hwang J. H. et al. (2015) [28] stated that there was no association between BMI and sudden SNHL. The reason for association between obesity and sudden SNHL could be due to obesity-associated microangiopathy in the vascular supply of cochlea, leading to pathological damage to inner ear [29]. In our study, out of 43 patients with sudden SNHL, 17 patients had hyperlipidaemia. The reason for sudden SNHL in these patients could be due to atherosclerosis in cochlear microvasculature due to elevated blood lipid levels.
In addition, the mean BMI of chronic rhinosinusitis group was 25.78 ± 2.33 kg/m2 which was significantly higher than mean BMI of control group. Bhattacharya N. et al. (2013) [30] and Chung S. D. et al. (2014) [31] also in their study showed increased association between chronic rhinosinusitis and obesity. The reason for association between obesity and chronic rhinosinusitis could be due to the changes in expression of obesity-linked cytokines, these cytokines being associated with chronic rhinosinusitis as well [32]. Besides this reason, in our study, we also found that 33 obese patients (32.3%) out of total 102 patients of group D with chronic rhinosinusitis also gave clinical history of GERD. Loehrl et al. (2012) [33] also suggested association between GERD and chronic rhinosinusitis. The reason could be due to impaired mucociliary clearance due to direct exposure of nasal mucosa to gastric acid [34]. However, there is very limited data support of this and needs further survey as both GERD and CRS are very commonly prevalent conditions, which could occur independently in a person as well.
In our study, the mean BMI was 25.03 ± 1.84 kg/m2 in group E (chronic tonsillitis), the difference between group E and control group (group F) being statistically significant (p = 0.004). This finding was consistent with observations made by Kim T. H. et al. (2015) [14] and Narang I. et al. (2012) [35]. Besides the known reason of obesity-associated cytokine expression, another reason for this could be mouth-breathing tendency among obese people and obesity-associated endocrine-mediated somatic growth, thus predisposing to recurrent larger tonsils.
Conclusion
Obesity leads to various ENT problems by altering the immune system. In our study, mean BMI was significantly higher in patients with otitis media effusion, chronic otitis media, chronic rhinosinusitis, sudden sensorineural hearing loss and chronic tonsillitis and also, as the severity of disease increased with increase in severity of BMI, showing positive correlation for all study groups, thus establishing association of obesity and these common otorhinolaryngological conditions. As obesity could be an important risk factor, early diagnosis and treatment of these ENT disorders is of prime importance in obese individuals.
Availability of data and materials
Available with corresponding author upon reasonable request.
References
Muller MJ, Geisler C (2017) Defining obesity as a disease. Eur J Clin Nutr 71:1256–1258
Pradeepa R, Anjana RM, Joshi SR (2015) Prevalence of generalised and abdominal obesity in urban and rural India: ICMR-INDIAB study (phase-I) [ICMR-INDIAB-3]. Indian J Med Res 142:139–150
World Health Organization Western Pacific Region (2000) The Asia-Pacific perspective: redefining obesity and its treatment. Health Communications Australia Pty Ltd, Sydney
Sturm R, Ringel JS, Andreyeva T (2004) Increasing obesity rates and disability trends. Health Aff (Millwood) 23(2):199–205
Monteiro R, Azevedo I (2010) Chronic inflammation in obesity and the metabolic syndrome. Mediators Inflamm 2010:289645
Krajewska J, Krajewski W, Zatonski T (2019) The association between ENT diseases and obesity in paediatric population: a systemic review of current knowledge. Ear Nose Throat J 98(5):E32–E43
Tchkonia T, Thomou T, Zhu Y, Karagiannides I, Pothoulakis C, Jensen MD et al (2013) Mechanisms and metabolic implications of regional differences among fat depots. Cell Metab 17(5):644–656
Lanza DC, Kennedy DW (1997) Adult rhinosinusitis defined. Otolaryngol Head Neck Surg 117:S1-7
Brodsky L (1989) Modern assessment of tonsils and adenoids. Paediatr Clin North Am 36(6):1551–1569
Heymsfield SB, Wadden TA (2017) Mechanisms, pathophysiology and management of obesity. N Engl J Med 376(15):1492
Heymsfield SB, Gonzalez MC, Shen W, Redman L, Thomas D (2014) Weight loss composition is one-fourth fat free mass: a critical review and of this widely cited rule. Obes Rev 15(4):310–321
Grant RW, Dixit VD (2015) Adipose tissue as an immunological organ. Obesity (Silver Spring) 23(3):512–518
Sidell D, Shapiro NL, Bhattacharyya N (2013) Obesity and the risk of chronic rhinosinusitis, allergic rhinitis and acute otitis media in school age children. Laryngoscope 123(10):2360–2363
Kim TH, Kang HM, Oh Ih, Yeo SG (2015) Relationship between otorhinolaryngological diseases and obesity. Clin Exp Otorhinolaryngol 8(3):194–197
Nam JS, Roh YH, Fahad WA, Noh HE, Ha JG, Yoon JH et al (2021) Association between obesity and chronic rhinosinusitis with nasal polyps: a national population based study. BMJ Open 11:e047230
Ahmed S, Arjmand E, Sidell D (2014) Role of obesity in otitis media in children. Curr Allergy Asthma rep 14(11):469
Kaya S, Selimoglu E, Cureoglu S, Selimoglu MA (2017) Relationship between chronic otitis media with effusion and overweight or obesity in children. J Laryngol Otol 131(10):866–870
Choi HG, Sim S, Kim SY, Lee HJ (2015) A high fat diet is associated with otitis media with effusion. Int J Paediatr Otorhinolaryngol 79(12):2327–2331
Yellon RF, Doyle WJ, Whiteside TL, Diven WF, March AR, Fireman P (1995) Cytokines, immunoglobulins and bacterial pathogens in middle ear effusions. Arch Otolaryngol Head Neck Surg 121(8):865–869
Bernstein JM (1996) Role of allegy in Eustachian tube blockage and otitis media with effusion: a review. Otolaryngol Head Neck Surg 114:562–568
Huang SL, Shiao G, Chou P (1999) Association between body mass index and allergy in teenage girls in Taiwan. Clin Exp Allergy 29(3):323–329
Sybilski AJ, Raciborski F, Lipiec A, Tomaszewska A, Lusawa A, Furmanczyk K et al (2015) Obesity- a risk factor for asthma, but not for atopic dermatitis, allergic rhinitis and sensitization. Public Health Nutr 18(3):530–536
Al-Saab F, Manoukian JJ, Al-Sabah B, Almot S, Nguyen LHP, Tewfik TL et al (2008) Linking laryngopharyngeal reflux to otitis media with effusion: pepsinogen study of adenoid tissue and middle ear fluid. J Otolaryngol Head Neck Surg 37:565–571
Rodrigues MM, Dibbern RS, Santos VJ, Passeri LA (2014) Influence of obesity on the correlation between laryngopharyngeal reflux and obstructive sleep apnoea. Braz J Otorhinolaryngol 80(1):5–10
MacArthur CJ, Pillers DA, Pang J, Kmepton JB, Trune DR (2011) Altered expression of middle and inner ear cytokines in mouse otitis media. Laryngoscope 121(2):365–371
Lee JS, Kim DH, Lee HJ, Kim HJ, Koo JW, Choi HG et al (2015) Lipid profiles and obesity as potential risk factors of sudden sensorineural hearing loss. PLoS One 10(4):e0122496
Lalwani AK, Katz K, Liu YH, Kim S, Weitzman M (2013) Obesity is associated with sensorineural hearing loss in adolescents. Laryngoscope 123:3178–3184
Hwang JH (2015) Role of obesity on the prognosis of sudden sensorineural hearing loss in adults. Otolaryngol Head Neck Surg 153(2):251–256
Jung SY, Shim HS, Hah YM, Kim SH, Yeo SG (2018) Association of metabolic syndrome with sudden hearing loss. JAMA Otolaryngol Head Neck Surg 144(4):308–314
Bhattacharyya N (2013) Associations between obesity and inflammatory sinonasal disorders. Laryngoscope 123(8):1840–1844
Chung SD, Chen PY, Lin HC, Hung SH (2014) Comorbidity profile of chronic rhinosinusitis: a population based study. Laryngoscope 124(7):1536–1541
Ghanim H, Aljada A, Hofmeyer D, Syed T, Mohanty P, Dandona P (2004) Circulating mononuclear cells in the obese are in a pro-inflammatory state. Circulation 110:1564–1571
Loehrl TA, Samuels TL, Poetker DM, Tochill RJ, Blumin JH, Johnston N (2012) The role of extraesophageal reflux in medically and surgically refractory rhinosinusitis. Laryngoscope 122:1425–1430
Delehaye E, Dore MP, Bozzo C, Mameli L, Delitala G, Meloni F (2009) Correlation between nasal mucociliary clearance time and gastroesophageal reflux disease: our experience on 50 patients. Auris Nasus Larynx 36:157–161
Narang I, Mathew JL (2012) Childhood obesity and obstructive sleep apnea. J Nutr Metab 2012:134202
Acknowledgements
None
Funding
Nil
Author information
Authors and Affiliations
Contributions
AS made contribution in design/writing of manuscript. AM and PK made contribution in data collection. AS and MM made contribution in statistical analysis. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted after approval by Institutional Ethics Committee of Government Medical College, Jammu. Written informed consent was taken from all subjects or their legal guardian in case of age of patient being less than 18 years.
Consent for publication
Written informed consent to publish patient’s clinical details was obtained from all subjects or their legal guardian in case of subject under 18 years.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saraf, A., Manhas, M., Manhas, A. et al. Obesity and ENT manifestations — a tertiary care centre study. Egypt J Otolaryngol 39, 22 (2023). https://doi.org/10.1186/s43163-023-00378-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43163-023-00378-3