Abstract
Background
Hearing loss in children constitutes a serious obstacle to their optimal development; early detection of hearing disability is vital to improve the outcome of management; currently, in Sudan, there is no national neonatal hearing screening program. The aim of this study is to discuss the results of neonatal hearing screening among newborns admitted to neonatal intensive care unit (NICU) and well-baby nursery, at Soba University Hospital, Khartoum (Sudan), and to reflect the magnitude of risk factors for hearing impairment present in these two different groups of newborns.
Methods
In this hospital-based cross-sectional study, newborns who were admitted to the NICU or being observed in the well-baby nursery in the period from February 2014 to February 2019 were screened for hearing loss using automated otoacoustic emission (OAE) device after recording the risk factors for hearing loss according to the Joint Committee on Infant Hearing (JCIH) year 2007 position statement and after conducting a detailed ear and general physical examination. Newborns who failed this screening test were rescreened using the same device after 48 h, and if they failed again, they underwent confirmatory auditory brainstem response test when they are 6 months old.
Results
One thousand one hundred twenty newborns were tested; 736 were NICU and 384 were well-baby nursery newborns. The prevalence of hearing impairment after confirmatory test was found to be 10.8 per 1000 in the NICU group and 5.2 per 1000 in the well-baby nursery group considering that the drop rate for the confirmatory test was 50% and 66% respectively which is very high and reflect the poor compliance to the program. The risk factors for hearing loss were more encountered in the NICU group compared to well-baby nursery group. Unmonitored ototoxic drug use was found to be very prevalent and need urgent reconsideration.
Conclusion
In this study, the estimated prevalence of neonatal hearing impairment is alarming; risk factors are very evident especially in the NICU group; this reflect the urgent need for establishing an efficient national program for neonatal hearing screening and working to eliminate the preventable risk factors for neonatal hearing impairment in this developing country.
Similar content being viewed by others
Background
Hearing loss is a major health problem worldwide; in young children, it constitutes a particular obstacle to their education and optimal development by delaying or even preventing them from acquiring speech, language, and cognitive skills. Early detection is vital to initiate immediate rehabilitation and management strategies during this critical period of central auditory pathway development [1]. The incidence of permanent bilateral hearing impairment is 1–2 out of 1000 live newborns and thus represents the most common sensory impairment in childhood [2].
Around 1.2 million children living in Sub-Saharan Africa (including Sudan) have a hearing impairment or hearing loss [3]. Within such countries, over 180,000 babies with a significant hearing loss are born annually [4].
Before the introduction of universal newborn hearing screening, the presence of a severe hearing impairment was detected at the age of 12 months on average due to the lack of first words formation. The diagnosis was made at a median age of 20 months and sometimes may be delayed until the time of school enrollment [5].
It has been shown that if intervention is initiated prior to 9 months of age, a child has the potential to develop and reach the same goals as others who do not have hearing loss [6,7,8].
Otoacoustic emission (OAE) test has become an essential component of objective pediatric audiology assessment. In humans, the otoacoustic emissions are sounds generated from the intact cochlea by oscillation (contraction and elongation) of the outer hair cells and transmitted across middle ear to the external ear where they can be recorded by a sensitive microphone and can be considered as a marker for inner ear integrity and a way to screen for hearing impairment. There are two types of OAE, the spontaneous (SOAE) which occur continuously without external stimuli and the transient evoked (TEOAEs) which is triggered by short external auditory stimuli (e.g., clicks, tone bursts) and can be measured in almost everyone with normal hearing.
TEOAE test is a safe technique for testing hearing from the middle ear to the level of the outer hair cells of the inner ear. TEOAE detection is a simple noninvasive technique, so it has become established as a standard screening method. It can detect a hearing loss of 20–30 dB in frequency bands of 1.5 and 4 kHz. This device have a sensitivity of 99.7% [9]. This screening tools needs confirmatory tests like ABR (auditory brainstem response), diagnostic OAE, and ASSR (auditory steady-state response) to confirm the diagnosis of hearing loss.
Although the etiology of congenital or early-onset hearing loss varies from country to country, there is widespread agreement that at least half of such hearing loss is due to genetic mutations. Adverse perinatal conditions (such as birth asphyxia, low birth weight and hyperbilirubinemia) and head trauma are also incriminated. In utero infections (such as cytomegalovirus, rubella, and meningitis), postnatal infections (such as measles, mumps and otitis media) can also cause hearing loss in infants and young children. More than half of the causes of hearing loss have been shown to be preventable and causes and risk factors related to hearing loss have been well documented [10]. The prevalence of hearing loss is higher within countries in Sub-Saharan Africa (including Sudan) because of difficulties in avoiding the preventable causes of hearing loss [11].
Methods
In this cross-sectional hospital-based prospective study, newborns delivered at Soba University Hospital, which is a tertiary hospital located in Khartoum (Sudan), in the period from February 2014 to February 2019, were screened for hearing loss using automated transient evoked otoacoustic emissions (TEOAEs) 24 h after delivery (to avoid the false positive results caused by ear canal debris found soon after birth); the model of the device we used is ERO.SCAN (MAICO), a portable, automated, battery operated, handheld with automatic probe fit. It has a frequency range of 3–5 kHz with a level measurement accuracy of ± 1 dB, dynamic range parameter 90 dB, has different sizes ear tips, present results as (PASS) or (REFER) form which can be printed, has noise alarm for noisy recording environment, and is easily calibrated. The test was conducted by a trained audiology nurse in a quiet room after obtaining a written consent from parents or caregivers, filling a datasheet containing contact information (residence, address, and phone number when available), personal data (gender and date of birth), and the assumed hearing loss risk factors (according to the Joint Committee on Infant Hearing regarding caregiver concern, NICU admission for more than 5 days with or without extracorporeal membrane oxygenation (ECMO), assisted ventilation, ototoxic drugs exposure, and jaundice that necessitate exchange transfusion). Besides in utero infection, craniofacial anomalies, syndrome associated with congenital hearing loss, and head trauma, we added other risk factors that are documented by other published literature to be associated with neonatal haring loss like consanguinity, family history of congenital hearing loss, maternal febrile illness, recurrent abortion, maternal exposure to ototoxic drugs, prematurity, Rhesus incompatibility, premature rupture of the membrane (PROM), delayed cry after delivery, and low birth weight and after conducting a detailed physical and otological examination by the authors. Newborns discharged before 24 h of delivery or did not attend the rescreening test in case they failed the first one were excluded due to the high incidence of false positive test in the first 24 h. Newborns who failed to pass the test were retested again using the same device after 48 h, and those who did not pass the test were sent for formal audiological assessment in the center outside the hospital using auditory brainstem response by experienced audiologist to confirm the diagnosis of permanent profound hearing loss at the age of 6 months.
Data analysis
Data was analyzed using Microsoft office Excel 2007; the mean and median age were calculated, and tables of risk factors and test results were produced; no analysis of variance or chi-square test were calculated because these are beyond the objectives of this study.
Results
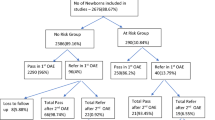
A total number of 1120 newborns were screened; 736 (65.7%) of them were (NICU), while 384 (34.3%) were well-baby nursery newborns; according to the exclusion criteria set, 785 newborns from the well-baby nursery who were discharged before 24 h of delivery were not included due to the high incidence of false positive in this age group.
Regarding the NICU group, the age at time of screening was found to range between 2 to 36 days, with mean of 4.5 days and median of 3 days. Four hundred fifteen of the newborns were males (56.3%), 320 (43.4%) were females, and one (0.3%) has undefined gender. Concerning the distribution of risk factors for hearing loss in the NICU group, 177 (24%) of the newborns were born to first- or second-degree consanguineous parents, 140 (19%) to third-degree consanguineous parents, and 419 (57%) to irrelative parents. Only 13 newborns (1.7%) had their parents admitted a positive family history of hearing loss, 22 newborns (2.9%) had their mothers admitted an exposure to ototoxic drugs, only one mother had developed fever and rash during pregnancy (0.1%), and eighteen (2.4%) of mothers had a history of recurrent abortion. The gestational age of tested newborns was found to range between 30 and 42 weeks, 153 newborn (20.7%) are between 37 and 33 weeks, while 45 (6.1%) were less than 33 weeks. In this group, Rhesus incompatibility was reported in 65 (8.8%) of newborns. Birth weight range was between 1.1 and 5.1 kg, with 14 newborns (1.9%) having a birth weight of ≤ 1.5 kg. One hundred twelve newborns (15.2%) did not cry immediately after birth indicating low Apgar score, and premature rupture of the membrane was evident in 54 newborns (7.3%). Ten newborns (1.35%) had febrile illness, six (0.8%) had rash, and five (0.67%) had convulsions; 115 newborns (15.6%) had jaundice, but this has necessitated exchange transfusion in only two (0.27%). Two hundred fifty-eight newborns (35%) received ototoxic drugs during their NICU admission for variable durations. Sixty-one newborns (8.2%) had dysmorphic features in the form of cleft palate in 5 (0.6%), hydrocephalus in 22 (3%), spina bifida in 11 (1.5%), Down syndrome in 4 (0.5%), microcephaly in one (0.1%), microphthalmia in one (0.1%), and other miscellaneous deformities in 17 (2.3%) (distribution of risk factors in the NICU group is shown in Table 1).
Of the 736 NICU newborns screened, 16 (2.17%) did not pass the screening TEOAE test in two occasions: 13 of them failed the screening test in both ears and 3 in one ear. Of the 16 newborns who did not pass the screening test, eight newborns did not attend the confirmatory ABR testing after 6 months (50% drop rate), sadly, four of them had passed away during hospitalization, and contact was lost with parents in case of the remaining four. The eight newborns tested were confirmed to have bilateral profound hearing loss (1.08%) using formal ABR by specialized audiologist; so, the overall estimated prevalence of hearing impairment is 10.8 per 1000 in the NICU group.
Regarding the 384 well-baby nursery newborns, the age range was between 2 and 19 days with a mean of 2.5 days and median of 2 days. Males were 188 (49%); females were 196 (51%). One hundred twelve (29.3%) of the newborns were born to first- or second-degree consanguineous parents, 90 (23.4%) to third-degree consanguineous parents, and 182 (47.3%) to irrelative parents. Seven newborns (1.8%) had family history of hearing loss, 42 (11%) had their mother exposed to ototoxic drug during pregnancy, 4 (1%) had their mothers exposed to febrile illness during pregnancy, 12 (3%) of mothers had history of recurrent abortion, and Rhesus incompatibility was present in 41 (10.6%) (the risk factors for hearing loss in this group are shown in Table 2).
Of the screened 384 well-baby nursery newborns, 6 (1.56%) did not pass the screening test in two occasions; all cases were bilateral. Of the six neonates who did not pass the test, four skipped the confirmatory test (66.6% drop rate), while two were confirmed to have bilateral significant hearing loss (0.52% overall); so, the overall estimated prevalence is 5.2 per 1000 (test results for both groups are shown in Table 3).
Discussion
Automated otoacoustic emission (OAE) is a reliable well-recognized hearing screening tool in newborns if conducted after the first 24 h of delivery; performing the test in the first hours is found to be associated with false (Refer) results due to debris accumulation in the ear canal. The policy adopted in our hospital is to discharge mothers 6 h after normal delivery, so the well-baby nursery group consists mainly of newborns delivered by caesarian section or had their mothers admitted to the hospital due to various medical causes; on the other hand, NICU newborns are ill and are usually hospitalized for long durations, so they are more in number in our study than that is expected for the normal ratio of NICU to the well-baby group. On the other hand, NICU group neonates are more exposed to risk factors that are associated with hearing loss.
The newborn age at time of screening has wide range in the NICU group with maximum of 36 days compared to 19 days in the well-baby group, while the median age is almost the same in the two groups; this delay in testing is due to the inability to test ill neonates until their general condition had allowed this.
Regarding the gender distribution, we found that males are more dominant in the NICU group while females are dominant in the well-baby nursery group; when calculating the male to female ratio in the well-baby nursery group before excluding newborns discharged in less than 24 h of delivery, the ratio is almost equal to that seen in the NICU group. An important risk factor for hearing impairment is the genetic predisposition; this can be reflected by both consanguinity and family history of congenital hearing loss; in our study, consanguinity is present in 43% of NICU and 53% of well-baby nursery newborns; this reflects social and cultural issues; Dror et al. concluded in their study that the degree of parental consanguinity is significantly and directly associated with hearing loss [12]. On the other hand, only 1.7–1.8% of the informers had admitted family history of hearing loss which is far less than the expected; this denial can be attributed to the social stigma of deafness in our society.
During prenatal period, there is marked exposure of mothers to ototoxic drugs and febrile infections; this indicates the poor antenatal care received by these mothers; the risk of neonatal hearing loss can be reduced if antenatal care is provided and encouraged; because congenital cytomegalovirus, toxoplasmosis and rubella are reportedly associated with late onset hearing impairment ( manifests during infancy or early childhood); hearing impairment due to these infections may not be detected during the neonatal period, so this necessitate the adoption of a follow-up hearing assessment program [13]; on the other hand, the recent introduction of rubella vaccination is expected to decrease the hearing affection related to rubella.
Additional risk factors peculiar to NICU group is prematurity (gestational age less than 37) which is a main indication for NICU admission and encountered in 20.7% of this group and low birth weight (≤ 1.5 kg) which was found in 1.9% of this group.
On the other hand, Rhesus incompatibility occurred in 8.8% of NICU neonates. Failure of neonates to cry immediately after birth is a known risk factor for hearing loss and was encountered in 15.2% of our NICU newborns. Febrile illness, rash, and convulsions were also observed and may potentiate the risk of hearing loss in our NICU series. Jaundice is evident in 15.6% of NICU neonates, but most of our cases were mild and did not necessitate exchange transfusion except in two cases (only 0.27%); hyperbilirubinemia requiring exchange blood transfusion is listed as a risk factor for hearing loss; this is consistent with reports from Nigeria, where the need for phototherapy exceeded exchange blood transfusion, but the Nigerian authors conclude that those who received phototherapy also being at significant risk for sensorineural hearing loss [14].
A serious risk factor is ototoxic drug exposure which is very prevalent in our NICU series accounting for 35% of neonates; it was considered in another study to be a major risk factor for hearing loss in newborns [15]. Ototoxic drugs are administered with no available serum levels measurement in our hospital; so, serum drug level monitoring or using alternative drugs will be expected to dramatically reduce the risk of neonatal hearing impairment.
Dysmorphism was observed in our NICU series in 8.3% of neonates screened; the most frequent conditions known to be associated with congenital hearing loss observed in our neonates were hydrocephalus which was encountered in 3%, cleft palate in 0.65%, Down syndrome in 0.5%, and microphthalmia in 0.1%. Premature rupture of membrane (PROM) is a well-recognized risk factor associated with neonatal hearing loss; it was observed in 7.3% of our NICU series.
It is important to recognize that cumulative effect of risk factors on hearing function is expected and needs further studies with suitable statistical analysis; the more risk factors present will increase the chance for the newborn to develop significant hearing impairment [16]; another vital point to be stressed on is that some of these risk factors are associated with delayed-onset hearing impairment which may not manifest until late infancy, so follow-up programs beside risk factors elimination initiatives are to be considered without delay.
The estimated prevalence of hearing loss in the NICU group in our study is 10.8 per 1000 neonate; due to the high drop rate (50%), this figure of course is just an approximation of the true prevalence; the failure of compliance to our screening protocol highlights the unawareness of our societies and even health system towards early detection of hearing impairment in neonates. Failure to confirm the diagnosis is due to the unavailability of the ABR device in the hospital, the cost, and sometimes the unawareness or even the refusal of the parents. On the other hand, the estimated prevalence of the well-baby nursery group is 5.2 per 1000 which is very high compared to the international figures (1–2 per thousand) [2]; although not accurate due to the high drop rate, this must attract the attention regarding the real magnitude of congenital hearing loss in Sudan among the low risk (apparently healthy) group; genetic predisposition (in form of consanguinity and family history of hearing loss) is a major risk factor in this group and needs universal hearing screening program together with genetic analysis. According to the reviewed literature, the prevalence for NICU is ten times higher than that in the well-baby nursery [17]; while it is only doubled in our study, this may indicate a high prevalence of hearing loss in otherwise healthy (low risk) neonates in our community.
Limitations
Total coverage of newborns delivered in our hospital was not possible because of the limited logistic and human resources and the exclusion of neonates who had been discharged before 24 h of delivery due to the high rate of false (Refer) results; this had made the accurate calculation of prevalence of haring loss a difficult task and also reversed the normal ratio of (NICU to well-baby nursery) newborn numbers. The drop rate was more than expected due to deficient compliance of parents to follow-up program. A more sophisticated statistical analysis is needed to correlate between the different risk factors and neonatal hearing impairment which was not an objective for the current study.
Conclusion
The calculated prevalence of neonatal hearing loss was 5.2 and 10.8 per 1000 in well-baby nursery and NICU respectively which is alarmingly high. The recognized risk factors observed in NICU are consanguinity, low birth weight, prematurity, congenital malformations, Rhesus incompatibility, premature rupture of membrane, antenatal ototoxicity, and maternal febrile illness. Unmonitored ototoxic drug use was found to be very prevalent and need urgent reconsideration. In the well-baby nursery group, the observed risk factors are genetic predisposition, Rhesus incompatibility, antenatal ototoxicity, and maternal febrile illness. The protocol of otoacoustic emission screening (conducted after the 24 h and repeated after 48 h if the newborn failed the test) confirmed by ABR at age of 6 months is a reliable tool for early detection of hearing loss in neonates. Awareness must be raised within medical practitioners, health providers, and parents regarding hearing screening, antenatal care, and risk factors of hearing loss.
Availability of data and materials
The datasets used and/or analyzed during the study are available from the corresponding authors on reasonable request.
Abbreviations
- NICU:
-
Neonatal intensive care unit
- OAE:
-
Otoacoustic emission
- JCIH:
-
Joint Committee on Infant Hearing
- TEOAEs:
-
Transient evoked otoacoustic emissions
- ABR:
-
Auditory brainstem response
- ASSR:
-
Auditory steady-state response
- SPSS:
-
Statistical Package for the Social Sciences
- PROM:
-
Premature rupture of membranes
References
Hilú MR, Zeigelboim BS (2007) The knowledge and valorization of neontal auditory screening and the early intervention of hearing loss. Rev CEFAC 9:563–570
Smith RJH, Shearer AE, Hildebrand MS (1999) Deafness and hereditary hearing loss overview. In: Pagon RA, Adam MP, Ardinger HH et al (eds) GeneReviews®. University of Washington, Seattle (WA), p 14
Tucci DL, Merson MH, Wilson BS (2010) A summary of the literature on global hearing impairment: current status and priorities for action. Otol Neurotol 31(1):31–41
Smith RJH, Bale JF, White KR (2005) Sensorineural hearing loss in children. Lancet 365(9462):879–890
Finckh-Krämer U, Spormann-Lagodzinski M, Gross M (2000) German registry for hearing loss in children: results after 4 years. Int J Pediatr Otorhinolaryngol 56(2):113–127
Nelson HD, Bougatsos C, Nygren P (2008) Universal newborn hearing screening: systematic review to update the 2001 US preventive services task force recommendation. Pediatrics 122(1)
Watkin P, McCann D, Law C, Mullee M, Petrou S, Stevenson J et al (2007) Language ability in children with permanent hearing impairment: the influence of early management and family participation. Pediatrics 120(3)
Yoshinaga-Itano C (2004) Levels of evidence: Universal newborn hearing screening (UNHS) and early hearing detection and intervention systems (EHDI). J Commun Disord 37(5):451–465
Neumann K, Indermark A (2012) Validation of a new TEOAE-AABR device for newborn hearing screening. Int J Audiol 51(8):570–575
Alberti PW (1996) The prevention of hearing loss worldwide. Scand Audiol Supplement 25(42):14–19
Abdalla FM, Omar MA (2011) The role of the health system in the prevention of hearing loss among children in Sub-Saharan Africa. Sudan J Paediatric 11(1):8–19
Dror AA, Avraham KB (2009) Hearing loss: mechanisms revealed by genetics and cell biology. Annu Rev Genet 43:411–437
Foulon I, Naessens A, Foulon W, Casteels A, Gordts F (2008) A 10-year prospective study of sensorineural hearing loss in children with congenital cytomegalovirus infection. J Pediatr 153:84–88
Olusanya BO, Somefun A (2009) Sensorineural hearing loss in infants with neonatal jaundice in Lagos: a community –based study. Ann Trop Paediatr 29(2):20–22
Kraft CT, Malhotra S, Boerst A, Thorne MC (2014) Risk indicators for congenital and delayed-onset hearing loss. Otol Neurotol 35:1839–1843
Martines F, Salvago P, Bentivegna D, Bartolone A, Dispenza F, Martines E (2012) Audiologic profile of infants at risk: experience of a Western Sicily tertiary care centre. Int J Pediatr Otorhinolaryngol 76:1285–1291
Erenberg A, Lemons J, Sia C, Trunkel D, Ziring P (1999) Newborn and infant hearing loss: detection and intervention. American Academy of Pediatrics. Task Force on Newborn and Infant Hearing, 1998–1999. Pediatrics 103:527–530
Acknowledgements
We are grateful to the dedicated audiology nurses, to the cooperative team of Soba NICU, and to the parents for allowing us to use the data of their newborns.
Funding
This study had received no funding.
Author information
Authors and Affiliations
Contributions
S.E.K designed the study and supervised the tests and conducted the ear examinations; E.M.O and A.E.M.K examined the newborns and facilitated the study in the NICU; N.A and O.M.K supervised the study and interpreted the results; all authors approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by Soba Center for Audit and Research (SCAR).
Written consents were obtained from caregivers of participants.
Consent for publication
Not applied
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kardman, S.E., Omer, E.M., Abdalla, N. et al. Neonatal hearing screening in Soba University Hospital, Khartoum, Sudan: a cross-sectional study. Egypt J Otolaryngol 39, 2 (2023). https://doi.org/10.1186/s43163-022-00372-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43163-022-00372-1