Abstract
Background
Parotid tumors are frequently encountered by otolaryngologists, and the vast majority of these are of the pleomorphic variety. Myoepitheliomas represent a small subset of parotid tumors. Due to their lack of specificity, they can easily be mistaken by an untrained eye for a pleomorphic adenoma. However, the recent advent of immunohistochemical staining of the tumors has aided in their diagnosis. These tumors show both benign and malignant potential; therefore, careful evaluation and subsequent appropriate management are warranted.
Case presentation
Here, we present a case of a young female patient who presented with a parotid lesion diagnosed as a case of myoepithelioma and dealt with accordingly. She underwent superficial parotidectomy under general anesthesia. A 2 × 2 cm firm nodular swelling was excised from the left parotid gland, and the facial nerve was identified and preserved.
Conclusions
The management of these lesions is usually centered on the surgeon’s experience and opinion as there have not been enough reported cases required for the formulation of an evidence-based guideline, hence more single-center studies are essential for evidence-based management.
Similar content being viewed by others
Background
Salivary glands represent a wide variety of cellular elements with varying ratios of constituents. Myoepitheliomas arise from myoepithelial cells and constitute a small fraction of salivary gland tumors [1]. They represent a fraction as small as 1–1.5% of all salivary gland tumors [2]. The identification of the category of the lesion, either benign or malignant, and the subsequent management have not been described extensively, thereby making it an entity that requires discussion and elaboration. Here, we have explained our experience with the pathology and have highlighted the major steps undertaken for its management.
Case presentation
A 19-year-old female presented to the Ear Nose and Throat (ENT) Clinic with a history of a small unilateral swelling over her left cheek for the past 3 months. There was no history of fever, local pain, sensory, or motor deficits of the face. On local examination, a 2 × 2 cm swelling localized to the pre-tragal region was noted. The swelling was normothermic to the surrounding tissue, firm, non-tender, and freely mobile with no overlying skin changes. No cervical lymphadenopathy was observed on examination. Neurological examination showed no signs of facial nerve compromise.
The patient was advised an ultrasound, which showed a 1.99 × 1.36 × 0.2 cm well-circumscribed solitary hypoechoic nodule in the left parotid gland with a few echogenic foci without any apparent lobulations. It was wider rather than tall and showed posterior acoustic enhancements likely representing a benign cystic lesion (Fig. 1A, B).
An ultrasound-guided fine-needle aspiration biopsy was performed. The smear picture showed small sheets of neoplastic cells with oval to spindle nuclei. The microscopic description characterized the lesion as cells arranged in sheets with focal tubule formation. No mitosis was seen with a positive reactivity for Cytokeratin AE1/AE3 and P63 and negative reactivity for desmin. A provisional diagnosis of salivary gland neoplasm with a myoepithelial component was given.
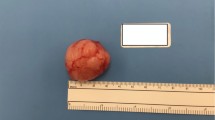
The patient was admitted for surgical management. She underwent superficial parotidectomy under general anesthesia. Following the standard preparation and draping, a left Blair incision was given, and a flap was raised anteriorly. A 2 × 2 cm firm nodular swelling was excised from the left parotid gland, with the facial nerve identified and preserved. The lesion was sent for histopathological analysis. Postoperatively, the patient remained vitally stable and facial nerve function was intact. A closed suction drain was placed. The drain was taken out subsequently on postoperative day one, and the patient was discharged. The patient was followed up in the clinic after 1 week, and no issues were observed at the first follow-up.
Histologically, the primary specimen showed a gray-white circumscribed lesion measuring 1.6 × 1 × 1 cm. On microscopic examination, the lesion showed focally infiltrative edges composed of epithelioid to polygonal cells with eosinophilic cytoplasm and centrally placed nuclei (Fig. 2A, B). Spindle cells or clear cells were scant. The cells were arranged in a trabecular arrangement with nuclei exhibiting mild atypia. On immunohistochemical staining, a diffuse pattern was observed with CKAE1/A3 and S100 stains along with patchy uptake for GFAP and actin (Figs. 3 and 4). The normal uptake of CKAE1/A3 was also visible in the normal parotid tissue as evident in Fig. 3A.
Discussion and conclusions
Secretory tissue across the body contains myoepithelial cells, including the lacrimal system, salivary glands, sweat glands, and mammary tissue. The initial reporting of the tumor, back in 1943, was associated with pleomorphic adenomas in a benign setting. However, the World Health Organization (WHO) categorized the tumor as a separate entity, distinguishing the pathology from pleomorphic adenomas in 1991 [1]. The two tumors were differentiated because of their increased number of cases and gross variations in ductular and myxo-vascular components with chondromyxoid stroma, and ductal structures found in pleomorphic adenomas but not in epithelioid myoepitheliomas; hence, the tumors were separately classified [3, 4].
Myoepitheliomas represent roughly 1% of the entire subset of parotid tumors which may arise de novo or within a pleomorphic adenoma [5]. The cases reported have shown a spectrum ranging from 20 to 60 years of age with a handful of cases reported in pediatric patients [6]. In our case, however, the tumor seemed to be arising de novo in a young female strengthening the idea of the non-specific epidemiological pattern. The tumor has shown both natures, benign and malignant. The malignant myoepitheliomas represent a very small subset of this tumor. In one meta-analysis, the malignant variant was shown to be as low as 7% in a background of 1% occurrence of myoepitheliomas amongst all parotid lesions [7].
The clinical presentation in most lesions gives a clue to invasiveness. The usual indicators of invasive parotid malignancy are facial nerve involvement and cervical lymph node extension [8]. In our case, the lesion had a short history with no signs of either local or regional extension. Like any other lesion, both radiological and histopathological analysis is warranted. Radiological investigations show a typically well-circumscribed and either smooth or lobulated lesion. The malignant variant can have nodal extension in the neck in radiology as well [9]. In our case, the lesion showed typical features like a cystic, well-circumscribed appearance and the dimensions supported a benign outlook.
The ultimate diagnosis, however, is based on histopathological evidence. Myoepitheliomas are characterized into four subtypes based on the cellular build-up. These include the spindle variety (65%), plasmacytoid (20%), epithelioid, and clear. The last two cell morphologies account for the remaining 15% [10]. In our case, the lesion constituted of polygonal cells with eosinophilic cytoplasm and centrally placed nuclei representing the epithelioid variety. The characterization of malignant transformation is based on features including nuclear atypia, mitotic activity, and cellular pleomorphism [9]. The diagnosis of myoepitheliomas is further strengthened by immunohistochemical staining. The stains usually showing reactivity in such tumors include glial fibrillary acidic protein (GFAP), Vimentin, S100, Calponin, CD10, cytokeratin, and p63 expression [8]. In our case, mild atypia with scant mitotic activity was observed with a varied response with staining. Patchy uptake was seen with ASMA, p63, and GFAP staining, whereas diffuse positivity was observed with Cytokeratin AE1/AE3 and S-100.
The management of these lesions is usually centered on the surgeon’s experience and opinion as there have not been enough reported cases required for the formulation of an evidence-based guideline. A small subset of lesions has a pre-surgical confirmation of the myoepithelial nature of the tumor. Consequently, with consideration for facial nerve involvement, superficial parotidectomy is the preferred approach [5]. In our case, the tumor was non-infiltrative and had no extension into the underlying nerve, so a superficial parotidectomy was done.
Post-operatively chemo-radiation can be considered for malignant lesions. However, the literature has not clearly shown any efficacy [5]. There is evidence of recurrence, however, amounting to roughly 15–18% of cases, and malignant transformation has also been reported in some cases as well [10].
Myoepitheliomas are usually benign tumors that can pose a diagnostic challenge for histopathologists, particularly in differentiation from pleomorphic adenomas due to their rarity and secondly in the categorization of malignant potential. The management is in turn based on this characterization, and hence, larger studies and reviews consisting of multiple cases are essential for determining the best evidence-based management.
Availability of data and materials
Not applicable
References
Simpson RH, Jones H, Beasley P (1995) Benign myoepithelioma of the salivary glands: a true entity? Histopathology. 27(1):1–9
Politi M, Toro C, Zerman N, Mariuzzi L, Robiony M (2005) Myoepithelioma of the parotid gland: case report and review of literature. Oral Oncol Extra. 41(6):104–108
Kawashima Y, Kobayashi D, Ishikawa N, Kishimoto S (2002) A case of myoepithelioma arising in an accessory parotid gland. J Laryngol Otol 116(6):474–476
Mochizuki Y, Omura K, Tanaka K, Sakamoto K, Yamaguchi A (2013) Myoepithelioma of the parotid gland presenting as a retroauricular cutaneous nodule: a case report. J Clin Diagnostic Res 7(6):1165
Morinière S, Robier A, Machet MC, Beutter P, Lescanne E (2003) Massive infra-clinic invasion of the facial nerve by a myoepithelial carcinoma of the parotid. Int J Pediatr Otorhinolaryngol 67(6):663–667
Monzen Y, Fukushima N, Fukuhara T (2007) Myoepithelioma and malignant myoepithelioma arising from the salivary gland: computed tomography and magnetic resonance findings. Aust Radiol 51:B169–B172
Nayak JV, Molina JT, Smith JC, Branstetter BF IV, Hunt JL, Snyderman CH (2003) Myoepithelial neoplasia of the submandibular gland: case report and therapeutic considerations. Arch Otolaryngol Head Neck Surg 129(3):359–362
Barca I, Novembre D, Cordaro R, Faro CL, Colangeli W, Boschetti CE, Giudice A, Cristofaro MG (2020) Myoepithelioma of the parotid gland: A case report with review of the literature. Oral and Maxillofacial Surgery Cases. 6(1):100131
Yaman H, Gerek M, Tosun F, Deveci S, Kılıç E, Arslan HH (2010) Myoepithelioma of the parotid gland in a child: a case report. J Pediatr Surg 45(7):e5–e7
Weitzel M, Cohn JE, Spector H (2017) Myoepithelioma of the Parotid Gland: A Case Report with Review of the Literature and Classic Histopathology. Case Rep Otolaryngol 2017:6036179. https://doi.org/10.1155/2017/6036179
Acknowledgements
None to declare
Funding
None to declare
Author information
Authors and Affiliations
Contributions
ABSV—Conception and design of the review, acquisition of the data, and analysis and interpretation of the data. MHD—Acquisition of data, analysis and interpretation of the data, and drafting of the article. MI—Conception and design of the review, final approval, and guarantor of the manuscript. MOA—Analysis and interpretation of the data and drafting of the article. NK—Acquisition of data, final approval, and guarantor of the manuscript. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval was provided by AKU–ERC Committee, and written informed consent was obtained from the patient for participation in this study.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vardag, A.B.S., Danish, M.H., Ikram, M. et al. Epithelioid myoepithelioma of the parotid gland: a case report with a brief literature overview. Egypt J Otolaryngol 38, 60 (2022). https://doi.org/10.1186/s43163-022-00237-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43163-022-00237-7