Abstract
Background
Variceal hemorrhage from the rupture of esophageal varices is accompanied by a substantial mortality rate. So, newly diagnosed cirrhotic patients are recommended to perform screening esophago-gastroduodenoscopy (EGD) for identifying varices. The primary objective of the present research was to ascertain the most precise biochemical and ultrasonographic variables that have the potential to non-invasively forewarn the occurrence of varices in cirrhotic patients. The study evaluated different parameters such as aspartate aminotransferase-to-platelet ratio index (APRI), platelet count/splenic diameter (PC/SD), portal vein velocity (PVV), and splenic and hepatic stiffness in prediction of EV.
Methods
This is a cross-sectional study that was conducted on 100 cirrhotic patients based on clinical, laboratory, and radiological assessments. All patients were subjected to thorough clinical examinations; laboratory tests were conducted to assess liver function and calculate Child–Pugh score and non-invasive tests for detecting esophageal varices such as APRI, PC/SD, Doppler ultrasonography for assessment of PV Doppler, and hepatic and splenic elastography. All patients got an endoscopic assessment in order to examine and classify the esophageal varices.
Results
Based on the current study, we found that predictors for EV among the studied patients were the following: PC/SD ratio with odds ratio (OR) was 2.20, PVV with OR was 4.68, liver stiffness with OR was 1.99, and splenic stiffness with OR was 3.55.
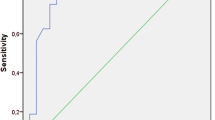
With ROC curve analysis, PVV has the best overall accuracy (85.4%) for prediction of EV with an area under the curve of 0.79 at cutoff point < 7.09 (cm/s) followed by splenic stiffness that has 82.6% overall accuracy with an area under the curve of 0.71 at cutoff point > 62.22 kPa.
Conclusion
PVV and splenic stiffness measurement hold potential as non-invasive markers for predicting the presence of esophageal varices in individuals with liver cirrhosis. Moreover, these markers can also aid in predicting the occurrence of advanced esophageal varices.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Introduction
Esophageal varices are a typical side effect of chronic liver disease, especially due to portal hypertension. A high death rate is linked to the rupture of these varices. Accordingly, as per the most recent guidelines [1], individuals with recently diagnosed cirrhosis are advised to have screening esophagogastroduodenoscopy in order to detect varices [1].
However, many patients are unsuitable for general anesthesia prior to endoscopy because they have co-occurring respiratory or cardiovascular conditions. Moreover, this procedure’s invasiveness leads in high healthcare expenses and suffering for the patient. So, the development of non-invasive techniques that can precisely forecast the existence and extent of esophageal varices is therefore of great interest [2, 3].
When esophagogastroduodenoscopy is not accessible, the use of precise noninvasive technologies like abdominal ultrasound and biochemical predictors becomes critical. This method would be especially useful in predicting esophageal varices non-invasively in high-risk cirrhotic patients, reducing the requirement for invasive diagnostic endoscopy. Furthermore, needless endoscopy could be avoided in low-risk cirrhotic individuals. Despite numerous studies evaluating these predictors, the results have not been consistent [4].
Therefore, the development and implementation of reliable non-invasive tools for predicting esophageal varices would have significant implications in clinical practice.
The current study aimed to discover the most reliable biochemical and ultrasonographic criteria for non-invasively predicting the existence of OV in patients with liver cirrhosis. The study evaluated different parameters such as PC/SD [5, 6], PVV [7], thrombocytopenia [8], splenic and hepatic elastography in prediction of EV [9, 10], and APRI [11].
Methods
The Assiut Faculty of Medicine’s ethical committee accepted the study protocol on January 25, 2022 (IRB number 17101635). All patients gave written informed permission. The research findings were used solely for scientific purposes, with no extraneous objectives. The danger of infection during blood sampling or endoscopy was reduced by following strict aseptic and sterilization procedures.
This cross-sectional study was conducted at the Internal Medicine and Radiology Departments, in our university hospital, in the period from December 2021 to April 2023. The study included a cohort of one hundred individuals who were diagnosed with liver cirrhosis using a set of diagnostic criteria established based on clinical history, examination, laboratory results, and sonographic criteria. The sonographic criteria indicative of the presence of liver cirrhosis encompass the following aspects: an increase in liver echogenicity, irregularities in liver margins, attenuation of intrahepatic portal and hepatic veins, and a relative enlargement of the caudate lobe and atrophy of the right lobe [1]. Upper endoscopy was done for them for detecting and grading of esophageal varices according to Westaby classification [12] into the following: grade I—varices appearing as slight protrusion above mucosa, which can be depressed with insufflations; grade II—varices occupying < 50% of the lumen; and grade III—varices occupying > 50% of the lumen which are very close to each other with confluent appearance.
The exclusion criteria encompassed individuals afflicted with hepato-cellular carcinomas (HCCs), portal vein thrombosis, massive ascites, hepatorenal syndrome, patients who underwent urgent esophageal varices band ligation in the same screening upper endoscopy session before PV Doppler assessment, and recent acute upper or lower gastrointestinal tract bleeding accompanied by hemodynamic compromise. Patients who exhibited positive viral serology, yet did not meet the diagnostic criteria for liver cirrhosis, were likewise excluded from the study.
Based on the accessibility of resources, the prerequisites of the proposed analysis plan, and the pervasiveness of hepatic cirrhosis in Upper Egypt, encompassing Assiut Governorate, the statistical assessment of sample size indicated that a population of 100 individuals receiving medical care at Assiut University Hospital would adequately reflect the requisite sample size for undertaking such a study, using the Epi Info software, considering the power (% chance of detection) of 80% and alpha error within 5%.
All enrolled patients underwent the following:
-
Comprehensive collection of medical background information: with particular emphasis on the history of viral hepatitis or exposure to risk factors (such as IV drug addicts, or previous surgical interventions not performed with complete aseptic techniques), history of jaundice, or bleeding tendency to bleed
-
Thorough clinical examinations: to identify signs of chronic liver disease and/or liver cell failure, which may include jaundice, palmer erythema, flapping tremors, spider-like blood vessels, bilateral lower limbs edema, hepatosplenomegaly, and ascites
-
Laboratory tests were conducted to assess liver function and calculate Child–Pugh score, including alanine transaminase (ALT), aspartate aminotransferase (AST), total bilirubin, serum protein and albumin levels, prothrombin time and concentration, and complete blood count. Kidney function was evaluated through tests measuring serum creatinine and blood urea levels.
Non-invasive methods for detecting esophageal varices
Aspartate aminotransferase-to-platelet ratio index (APRI)
It is a blood test used to assess liver fibrosis in chronic hepatitis C patients, although it does not directly indicate the existence of EV [11].
Platelet count/spleen diameter ratio (PC/SD)
An ultrasound scan was employed to assess the maximum splenic bipolar diameter. This measurement was presented in millimeters (mm). Additionally, the platelet count was expressed as PLT (number of platelets per cubic millimeter). This ratio is used as an indicator of the severity of portal hypertension, with lower ratios suggesting more significant portal hypertension [13].
Abdominal ultrasonography, PV Doppler, and hepatic and splenic elastography
These were conducted by a single experienced radiologist using Logiq P9 ultrasound machine (GE healthcare; USA) with a 3.5-MHz convex probe equipped with 2D shear wave elastography (2D-SWE) function. The focus of abdominal US was on identifying the criteria of chronic liver disease and cirrhosis, detecting ascites, and measuring the size of the spleen.
PV Doppler
A portal vein Doppler ultrasound was conducted in the early morning while fasting to mitigate the potential variations in portal pressure and minimize gas and bowel activity. The portal vein was visualized in a longitudinal manner while the person is lying supine. Subsequently, the velocity of blood flow in the portal vein (PVV) and the diameter of the portal vein were determined. The scanner’s position was fine-tuned to obtain a Doppler angle of less than 60°. The Doppler sample volume was placed at the point where the portal vein crosses with the hepatic artery and was adjusted to the middle of the portal vein. The average velocity of the blood flow in the portal vein was 15–30 cm/s.
For hepatic elastography measurement
The SWE box was positioned in the right lobe of the liver, specifically 1.5–2 cm below and perpendicular to the liver capsule. This was done using the intercostal approach, which involves inserting the box parallel to the rib space.
For splenic elastography measurement
Splenic elastography was assessed 1 cm below the capsule using a left-sided intercostal technique. The velocity is expressed in meters per second and then automatically converted to tissue stiffness, which is expressed in kilopascals. This conversion is done using the formula e = 3 ρ v2, where e represents tissue elasticity, ρ represents tissue density, and v represents shear wave velocity. Each patient underwent ten measures for their liver and spleen, which were deemed reliable if the ratio of the interquartile range to the median (IQR/m) was less than 30%.
Statistical analysis of the data
Statistical analysis was performed on the data using SPSS (Statistical Package for the Social Sciences, version 20, IBM, New York). The continuous data was represented using the mean value plus or minus the standard deviation (SD), whereas the nominal data was represented using the frequency given as a percentage.
The chi-square test was employed to analyze the nominal data of several groups in the study, while Student’s t-test was utilized to compare the means of two separate groups. The predictors of esophageal varices in the patients under study were identified using logistic regression. The accuracy of several predictors in diagnosing esophageal varices was assessed using a receiver operating characteristic (ROC) curve (Fig. 1). A p value was deemed significant if it was less than 0.05.
Results
Characteristics of studied patients based on presence of EV
This study was conducted on one hundred patients. Among these individuals, there were sixty patients diagnosed with OVs. Only five patients had cherry red spot sign on the OVs in the screening upper endoscopy, and band ligation was done in another scheduled endoscopic session after performing PV Doppler assessment. It is worth noting that both groups of patients were carefully selected to ensure that they were comparable in terms of age and gender. Information regarding the demographics of the patients and the main clinical and laboratory finding can be found in Table 1. Both groups exhibited negligible dissimilarities as regards OVs and Child–Pugh classification.
Doppler ultrasound and elastography assessment in studied patients based on presence of EV (Table 2)
Patients with EV had significantly lower PVV (7.90 ± 2.35 vs. 16.56 ± 3.90 (cm/s); p < 0.001) in comparison with those without EV.
At the same time, patients with EV had significantly higher liver stiffness (49.11 ± 5.67 vs. 31.11 ± 8.45 (kPa); p = 0.03) and splenic stiffness (65.56 ± 15.44 vs. 41.56 ± 16.11 (kPa); p < 0.001) in comparison with those without EV.
Only 15 patients had gastric varices including gastroesophageal varices type I (n = 5) and II (n = 4) and isolated gastric varices (n = 6). There was no statistically significant difference between patients with gastric varices and those without gastric varices regarding splenic diameter, APRI, PC/SD ratio, PVV, and hepatic and splenic elastography (Table 3).
Different parameters in patients with varices based on its grades (Table 4)
A total of 20/60 (33.3%) patients with EV had grade I/II EV, while other 40/60 (66.7%) patients had grade III EV. Both groups of patients based on grades of EV had insignificant differences as regards splenic diameter or APRI, while patients with grade III EV had significantly lower PC/SD (760.98 ± 127.89 vs. 909.18 ± 123.50 (p < 0.001) and PVV (7.11 ± 2.01 vs. 10.22 ± 2.76 (cm/s); p < 0.001) with higher splenic stiffness (70.89 ± 3.11 vs. 55.50 ± 10.57 (kPa); p < 0.001) in comparison with those with grade I/II EV.
Regression analysis for prediction of esophageal varices in studied patients (Table 5)
According to the present study, we have identified that predictors for EV were as follows: PC/SD ratio with odds ratio (OR) was 2.20, PVV with OR was 4.68, liver stiffness with OR was 1.99, and splenic stiffness with OR was 3.55. With ROC curve analysis, PVV has the best overall accuracy (85.4%) for prediction of EV with an AUC of 0.79 at cutoff point < 7.09 (cm/s) followed by splenic stiffness that has 82.6% overall accuracy with an AUC of 0.71 at cutoff point > 62.22 kPa (Tables 5 and 6).
Discussion
Esophageal varices (EV) primarily result from portal hypertension, and their rupture is linked to a significant mortality rate. As per the latest guidelines, it is encouraged that all patients who have recently been diagnosed with cirrhosis should have screening esophago-gastroduodenoscopy (EGD) in order to detect varices [14].
Nevertheless, the intrusive characteristics of EGD result in substantial healthcare expenses and patient unease. Consequently, there is significant interest in creating non-invasive techniques that have satisfactory diagnostic precision for predicting the existence and dimensions of EV [4, 14, 15].
Multiple studies were conducted to detect accurate noninvasive predictors for detecting EV, but they yield inconsistent findings [16,17,18,19].
The objective of the current study was to determine the most precise biochemical and ultrasonographic indicators that can be used to predict the occurrence of EV patients with hepatic cirrhosis, without the need for invasive procedures. The study assessed various measures, with focusing on PVV and splenic and hepatic stiffness.
The study included 100 patients with cirrhotic patients, and based on upper endoscopy, 60 (60%) patients had EV (EV group), and 40 (40%) had no EV (No-EV group). Both groups had insignificant difference as regards baseline characteristics, Child–Pugh class, bilirubin level, and APRI score, while platelet count was significantly lower in the EV group, and liver enzymes were higher. This finding is incongruent with the research conducted by Arul Prakash et al. [20] and Mahmoud et al. [21], as they reported that patients with esophageal varices (OVs) had significantly higher levels of serum bilirubin compared to those without varices. However, our results align with their findings regarding the presence of thrombocytopenia in patients with esophageal varices (EV groups). Also, many previous studies reported low platelet counts among patients with EV [5, 22].
The splenic diameter exhibited elevated levels, while the PC/SD demonstrated decreased levels in the EV group (p < 0.001). These observations align with the findings of Baig et al. [23], El Makarem et al. [6], and Faheem et al. [24]. However, they are in disagreement with the studies conducted by Mahmoud et al. [21] and Mahran et al. [25]. The disparity can be accounted for by varying fundamental causes and levels of liver impairment among the two sets of investigations.
The study included 100 patients with positive HCV and based on upper endoscopy; 60 (60%) patients had EV (EV group), and 40 (40%) had no EV (No-EV group). Both groups had insignificant difference as regards baseline characteristics. Similarly, Stefanescu et al. studied a total 135 patients with LC. The authors found that 124 (84.4%) and 21 (15.6%) patients with and without EV, respectively, with no significant differences as regards patients’ characteristics [26].
Another study enrolled 180 cirrhotic patients; out of them, 22.7% of patients had normal endoscopy, and the other 87.8% of patients had EV. There were no significant differences between both groups as regards baseline characteristics [27]. Many previous studies were consistent with these findings [3, 24, 28].
Another finding in the current study was that both groups had insignificant differences as regards baseline laboratory data with exception of lower platelet count (122.43 ± 13.45 vs. 145.80 ± 10.45 (× 106 /ml); p = 0.04) among patients with EV. This was consistent with many previous studies that reported low platelet count among patients with EV [5, 21].
The pathogenesis of thrombocytopenia in liver cirrhosis is mainly linked to hypersplenism, where portal hypertension leads to pooling and sequestration of all corpuscular elements of the blood, predominantly thrombocytes in the enlarged spleen [8, 22, 29].
In addition, we found that patients with EV had higher splenic diameter (121.54 ± 21.45 vs. 139.87 ± 15.56 (mm); p < 0.001) and significantly lower PC/SD ratio (890.56 ± 155.09 vs. 1098.45 ± 234.56; p < 0.001). This was consistent with a previous study that reported patients with EV had higher splenic diameter (140 ± 4.33 vs. 158 ± 14.88 (mm); p < 0.001) with lower PC/SD ratio (506.367 ± 156.89 vs. 744.510 ± 60.18; p < 0.001) [24].
Furthermore, the current study showed that the PC/SD ratio was a predictor for EV in patients with liver disease with OR being 2.20 with an overall accuracy being 74.2% at cutoff point < 909. Similarly, in a study conducted by Faheem et al. [23], it was discovered that a PC/SD ratio of < 668.97 had a sensitivity of 86.49%, specificity of 100%, and accuracy of 90.1%. This highlights the significance of the PC/SD ratio in predicting the presence of esophageal varices [24].
El Makarem et al. reported that a cutoff value of 939.7 for this ratio resulted in a diagnosis accuracy of 96.5%. In addition, two previous studies have revealed comparable results. One study found that a PC/SD ratio of 1330 had a sensitivity of 76.9%, specificity of 84.3%, and accuracy of 83%. Another study found that a PC/SD ratio of < 833.3 had a sensitivity of 73.48%, specificity of 64.29%, and accuracy of 72.62% [30].
Our study revealed that patients with grade III EV exhibited a notable decrease in PC/SD (760.98 ± 127.89 vs. 909.18 ± 123.50 (p < 0.001)) in comparison with those with grade I/II EV. Yet, with regression analysis, PC/SD ratio was not a predictor for advanced EV. A previous study stated that this ratio at cutoff point < 425.9 had a sensitivity of 70% for prediction of advanced EV [2]. Many studies were consistent with the latter study [6, 12, 26, 31].
One of the main findings in the current study was that patients with EV demonstrated notably reduced PVV (7.90 ± 2.35 vs. 16.56 ± 3.90 (cm/s); p < 0.001) in comparison with those without EV. In addition, patients with grade III EV had significantly lower PVV (7.11 ± 2.01 vs. 10.22 ± 2.76 (cm/s); p < 0.001) with regression analysis; PVV was a predictor for the presence of EV with OR being 4.68 and predictor for advanced EV with OR being 4.11.
At the same time, PVV had the best overall accuracy (85.4%) for prediction of EV with an AUC of 0.79 at cutoff point < 7.09 (cm/s), and the best overall accuracy (89.3%) for prediction of grade III EV with AUC was 0.79 at cutoff point < 6.99 (cm/s). Similarly; Elkenawy et al. stated lower values of PVV in variceal patients compared to non-variceal patients (p value = 0.000); moreover, the PVV showed a considerable decrease in grade III when compared to grade I/II EV [32].
The latter study also highlighted the potential utilization of portal vein velocity (PVV) as a rapid noninvasive screening predictor for EV, presenting an intriguing prospect within the realm of clinical practice. This proposition is underscored by the finding in the study demonstrating its high diagnostic accuracy, as evidenced by an impressive area under the receiver operating characteristic curve (AUROC) of 0.927. Specifically, the establishment of a cutoff value of less than 7 cm/s for PVV demonstrates remarkable sensitivity, reaching 97%, coupled with a notable odds ratio of 16.50. Furthermore, the results emphasized that other examined noninvasive predictor have less prediction accuracy relative to PVV [32].
Our results also were confirmed by previous studies that proved that PVV could be used as noninvasive triage tests before referral to endoscopy [33,34,35]. A previous study concluded that PVV has the highest sensitivity of 84% at a cutoff level of 16 cm/s in comparison with other parameters [34]. Moreover, Kayacetin et al. concluded that PVV decreased with the severity of liver cirrhosis and may predict variceal bleeding risk [33].
In a more recent study in 2023, 137 cirrhotic patients were enrolled. The authors found that PVV was a predictor for prediction EV. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and diagnostic accuracy for esophageal varices were determined to be 93.83%, 92.86%, 95%, 91.23%, and 93.43%, respectively, using a cut-off value of 18 cm/s for PVV [7]. Also, at cutoff point < 19 cm/s, another study stated a 93.4% accuracy in prediction of EV [36].
Another finding in the current study was that liver and splenic stiffness were significantly higher among patients with EV. In addition, both of them were predictors for the presence of EV with OR being 1.99 (liver stiffness) and 3.55 (splenic stiffness), respectively. It was found that splenic stiffness had superior diagnostic accuracy over liver stiffness for prediction of EV (82.6% vs. 61.2%, respectively) at cutoff point of > 41.11 and 62.22 kPa, respectively.
At the same time, we found that liver stiffness (LS) showed no significant differences between patients with grade III and those with grade I/II EV. Meanwhile, splenic stiffness was significantly higher among patients with grade III EV. At cutoff point > 65.45 kPa, splenic stiffness (SS) had 84% overall accuracy in prediction of grade III EV with odds ratio being 3.23.
In recent times, an increasing number of trials have endeavored to elucidate the efficacy of splenic stiffness (SS) and liver stiffness (LS) in diagnosing esophageal varices (EV) among patients with chronic liver disease (CLD). However, findings from these studies have yielded contentious outcomes. Specifically, research has indicated that SS emerges as a superior parameter exhibiting high diagnostic accuracy in the identification and grading of EV when compared to LS [9].
Conversely, other studies have concluded that spleen elastography is not superior to liver elastography in predicting EV for its inconstant accuracy, poor repeatability, and highly unreliable measurement [10, 37, 38].
A meta-analysis comprising 16 studies and involving 1892 patients concluded that spleen stiffness (SS) surpasses liver stiffness (LS) in its predictive capability for detecting the presence of esophageal varices (EV). Despite the relatively modest accuracy of both parameters in discerning severe cases of EV, they remain viable options for screening EV in individuals newly diagnosed with cirrhosis. In detection of any EV, for LS, the summary sensitivity was 83%, and the specificity was 66% [39]. The sensitivity and specificity of spleen stiffness (SS) measurement were calculated to be 88% and 78%, respectively. The overall receiver operating characteristic (ROC) curve values for liver stiffness (LS) and SS were found to be 0.81 and 0.88, respectively. These results were statistically significant with a p value of less than 0.01. The odds ratio of SS (25.73) was significantly higher than that of LS (9.54), with the relative odds ratio value being 2.48 (95% CI: 1.10–5.60; p < 0.05) [39].
González-Ojeda et al. stated that the best cut-off for SS was 36.3 kPa, and it was 48 kPa for LS. The area under the SS and LS ROC curves in predicting esophageal varices were 0.66 and 0.51, respectively. The sensitivity and specificity of SS for EV occurrence were 63.9 and 68.4, respectively. The sensitivity and specificity of LS for EV occurrence were 83.3 and 26.3, respectively [22].
Previous meta-analysis stated that SS was superior to LS for detection of EV with higher sensitivity (0.90 and vs 0.85), specificity (0.73 vs 0.64), OR (3.24 vs 2.26), and AUC (0.899 vs 0.817). For detection of advanced EV, SS had the highest sensitivity (0.87) followed by LS (0.85) [40].
We noticed that there was a wide range in different best cutoff points of different studied parameters between different studies including our study; this could be explained by different sample size, selection bias, studied population, the subjective assessment of the size of esophageal varices on endoscopy, and the unequal distribution of patients according to the EV grade, leading to differently sized subgroups of patients. Also, the selection criteria in different studies were not the same as in our study; we enrolled only patients with HCV infection, and other studies enrolled only patients with HBV infection [19].
Based on the current study, it is apparent that these robust sensitivity and odds ratio metrics suggest that potential utilization of both portal vein velocity (PVV) and splenic stiffness measurements hold promise in discerning EV presence, thus providing clinicians with a dependable and efficient screening tool for this clinically significant condition. Furthermore, these measurements exhibit potential utility in predicting advanced stages of esophageal varices, particularly grade III EV.
Our research was constrained by two primary limitations. The first limitation was the few number of patients with cherry red spot sign included, as these patients required urgent band ligation, which could affect the measurement of portal vein velocity. The second limitation in our study was the small number of patients with gastric varices especially those with isolated fundal varix. So, further research about usefulness of non-invasive parameters is recommended in a larger sample size of patients with cherry red spot sign and gastric varices.
Availability of data and materials
Data of the previous cases in this manuscript are present.
Abbreviations
- ALT:
-
Alanine transaminase
- AST:
-
Aspartate aminotransferase
- APRI:
-
Aspartate aminotransferase-to-platelet ratio index
- AUC:
-
Area under the curve
- CLD:
-
Chronic liver disease
- EGD:
-
Esophago-gastroduodenoscopy
- EV:
-
Esophageal varices
- HCCs:
-
Hepato-cellular carcinomas
- IQR/m:
-
Interquartile range to the median
- NPV:
-
Negative predictive value
- OR:
-
Odds ratio
- PC/SD:
-
Platelet count/splenic diameter
- PVV:
-
Portal vein velocity
- PPV:
-
Positive predictive value
- 2D-SWE:
-
2D shear wave elastography
- ROC:
-
Receiver operating characteristic
- SPSS:
-
Statistical Package for the Social Sciences
- SD:
-
Standard deviation
- SS:
-
Spleen stiffness
References
Bates JA (2004) Abdominal ultrasound. Int J Acad Med Pharm 8(2):65–69
Anbukumar T, Ramesh N, Subbiah J, Babu CN (2023) A study of non-invasive predictors of esophageal varices in patients with chronic liver disease in a tertiary care hospital. Int J Acad Med Pharm 5(2):172–178
Ashraf DG, El-Sayed I (2018) Esophageal varices predictive score in liver cirrhosis. Egypt J Intern Med 30:72–77
Berzigotti A, Bosch J, Boyer TD (2014) Use of noninvasive markers of portal hypertension and timing of screening endoscopy for gastroesophageal varices in patients with chronic liver disease. Hepatology 59(2):729–731
Chawla S, Katz A, Attar BM, Gupta A, Sandhu DS, Agarwal R (2012) Platelet count/spleen diameter ratio to predict the presence of esophageal varices in patients with cirrhosis: a systematic review. Eur J Gastroenterol Hepatol 24(4):431–436
El Makarem MAA, Shatat ME, Shaker Y, Aleem AAA, El Sherif AM, Moaty MA et al (2011) Platelet count/bipolar spleen diameter ratio for the prediction of esophageal varices: the special Egyptian situation: noninvasive prediction of esophageal varices. Hepat Mon 11(4):278
Khan HMW, Bilal B, Khan K, Butt MOT, Shah AA, Aujla UI et al (2023) Diagnostic accuracy of portal vein flow velocity for esophageal varices in cirrhotic patients. Cureus 15(8):e43592
Nilles KM, Flamm SL (2020) Thrombocytopenia in chronic liver disease: new management strategies. Clin Liver Dis 24(3):437–451
Takuma Y, Nouso K, Morimoto Y, Tomokuni J, Sahara A, Toshikuni N et al (2013) Measurement of spleen stiffness by acoustic radiation force impulse imaging identifies cirrhotic patients with esophageal varices. Gastroenterology 144(1):92-101. e2
Zykus R, Jonaitis L, Petrenkienė V, Pranculis A, Kupčinskas L (2015) Liver and spleen transient elastography predicts portal hypertension in patients with chronic liver disease: a prospective cohort study. BMC Gastroenterol 15:1–7
Wai C-T, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS et al (2003) A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 38(2):518–526
Westaby D, Macdougall BR, Melia W, Theodossi A, Williams R (1983) A prospective randomized study of two sclerotherapy techniques for esophageal varices. Hepatology 3(5):681–684
Giannini E, Botta F, Borro P, Risso D, Romagnoli P, Fasoli A et al (2003) Platelet count/spleen diameter ratio: proposal and validation of a non-invasive parameter to predict the presence of oesophageal varices in patients with liver cirrhosis. Gut 52(8):1200
Glisic T, StojkovicLalosevic M, Milovanovic T, Rankovic I, Stojanovic M, Toplicanin A et al (2022) Diagnostic value of non-invasive scoring systems in the prediction of esophageal varices in patients with liver cirrhosis—single center experience. Medicina 58(2):158
Colecchia A, Ravaioli F, Marasco G, Colli A, Dajti E, Di Biase AR et al (2018) A combined model based on spleen stiffness measurement and Baveno VI criteria to rule out high-risk varices in advanced chronic liver disease. J Hepatol 69(2):308–317
Cifci S, Ekmen N (2021) Evaluation of non-invasive fibrosis markers in predicting esophageal variceal bleeding. Clin Endosc 54(6):857–863
Li S, Huang P, Jeyarajan AJ, Ma C, Zhu K, Zhu C et al (2021) Assessment of non-invasive markers for the prediction of esophageal variceal hemorrhage. Front Med 8:770836
Xian-guang H, Song-hu L, Mei-ting H, Guang-yao W, Fu Z, Xiao-hong C (2020) Prediction of esophageal variceal bleeding in patients with hepatic cirrhosis by different non-invasive markers. Chin Hepatol 25(7):724
Yu S, Chen W, Jiang Z (2021) Platelet count/spleen volume ratio has a good predictive value for esophageal varices in patients with hepatitis B liver cirrhosis. PLoS ONE 16(12):e0260774
Arul Prakash S, Shanmugam C, Kalyanasundaram M, Rangachari B, Thangavelu P, Subbarayan J (2010) Noninvasive prediction of large esophageal varices in chronic liver disease patients. Saudi J Gastroenterol 16(1):38–42
Mahmoud H, Mostafa E, Mohammed M (2014) Role of portal haemodynamic parameters in prediction of oesophageal varices in cirrhotic patients. Arab J Gastroenterol 15(3):130–134
González-Ojeda A, Cervantes-Guevara G, Chávez-Sánchez M, Dávalos-Cobián C, Ornelas-Cázares S, Macías-Amezcua MD et al (2014) Platelet count/spleen diameter ratio to predict esophageal varices in Mexican patients with hepatic cirrhosis. World J Gastroenterol 20(8):2079
Baig W, Nagaraja M, Varma M, Prabhu R (2008) Platelet count to spleen diameter ratio for the diagnosis of esophageal varices: is it feasible? Can J Gastroenterol Hepatol 22(6):825–828
Faheem HA, Mohamed MA, Eid EE, Said NM (2022) Value of non-invasive scores and modalities in predicting the presence of esophageal varices in patients with liver cirrhosis. Egypt J Hosp Med 88(1):2464–2471
Mahran Z, Ibrahim A, Abdel GS (2006) Non-invasive prediction of esophageal varices in chronic liver disease patients: a Doppler study. Sc J Az Med Fac (Girls) 27(3):969–980
Stefanescu H, Grigorescu M, Lupsor M, Procopet B, Maniu A, Badea R (2011) Spleen stiffness measurement using Fibroscan for the noninvasive assessment of esophageal varices in liver cirrhosis patients. J Gastroenterol Hepatoly 26(1):164–170
Mathur P, Rajender A, Choudhary P, Gupta D, Rijhwani P, Saxena GN (2020) Sensitivity and specificity of platelet count/splenic diameter ratio for prediction of oesophageal varices in Indian cirrhotics. Alcoholism 119:66.1
Elatty EA, Elshayeb EI, Badr MH, Mousa WA, El Mansory MF (2019) Noninvasive parameters for assessment of esophageal varices. Egypt J Intern Med 31:536–543
Scharf RE (2021) Thrombocytopenia and hemostatic changes in acute and chronic liver disease: pathophysiology, clinical and laboratory features, and management. J Clin Med 10(7):1530
Shibata S, Joshita S, Umemura T, Yamazaki T, Fujimori N, Ichikawa Y et al (2016) Liver stiffness-spleen size-to-platelet ratio risk score detects esophageal varices in chronic liver disease. Springerplus 5(1):1–6
Cherian JV, Deepak N, Ponnusamy RP, Somasundaram A, Jayanthi V (2011) Non-invasive predictors of esophageal varices. Saudi J Gastroenterol 17(1):64
Elkenawy YN, Elarabawy RA, Ahmed LM, Elsawy AA (2020) Portal vein flow velocity as a possible fast noninvasive screening tool for esophageal varices in cirrhotic patients. JGH Open 4(4):589–594
Kayacetin E, Efe D, Doğan C (2004) Portal and splenic hemodynamics in cirrhotic patients: relationship between esophageal variceal bleeding and the severity of hepatic failure. J Gastroenterol 39:661–667
Shastri M, Kulkarni S, Patell R, Jasdanwala S (2014) Portal vein Doppler: a tool for non-invasive prediction of esophageal varices in cirrhosis. J Clin Diagn Res 8(7):112
Wicaksono KP, Matondang S, Silman C, Prihartono J, Lesmana CRA (2022) A novel splenic vein flow volume to the portal vein flow velocity index as a predictor for the degree of esophageal varices in liver cirrhosis patients. Case Rep Gastroenterol 16(1):179–185
Katwal S, Ansari MA, Suwal S, Rayamajhi S, Ghimire P, Ghimire A (2023) Measurement of portal vein indices and splenic index by ultrasound and their association with gastroesophageal varices in cirrhosis of liver. Ann Med Surg 85(12):5926–5931
Calvaruso V, Di Marco V, Bronte F, Licata G, Simone F, Butera G et al (2010) 388 spleen stiffness correlates with portal hypertension and increases the accuracy of detection of esophageal varices in HCV cirrhosis. J Hepatol 52:S159–S160
Procopet B, Berzigotti A, Abraldes JG, Turon F, Hernandez-Gea V, García-Pagán JC et al (2015) Real-time shear-wave elastography: applicability, reliability and accuracy for clinically significant portal hypertension. J Hepatol 62(5):1068–1075
Ma X, Wang L, Wu H, Feng Y, Han X, Bu H et al (2016) Spleen stiffness is superior to liver stiffness for predicting esophageal varices in chronic liver disease: a meta-analysis. PLoS ONE 11(11):e0165786
Manatsathit W, Samant H, Kapur S, Ingviya T, Esmadi M, Wijarnpreecha K et al (2018) Accuracy of liver stiffness, spleen stiffness, and LS-spleen diameter to platelet ratio score in detection of esophageal varices: systemic review and meta-analysis. J Gastroenterol Hepatol 33(10):1696–1706
Acknowledgements
The authors thank the Gastroenterology Unit of Internal Medicine Department and members of the Radiology Department, Assiut University, doctors, and the nursing team for the clinical care of patients included in this work.
Funding
No fund or sources of support from any committee.
Author information
Authors and Affiliations
Contributions
Abdul-Allah Ismael Kelany: conception and supervision of the work. Ramy Mohamed Ahmed: performing Doppler ultrasound and elastography. Salma Mokhtar Osman Hassan and Khaled Mohamed Ali Shehata: drafting of the manuscript, supervision of the work. Peter Atef Munir: collection of data.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study is in accordance with the Declaration of Helsinki and approved by the appropriate ethical committee of the Faculty of Medicine, Assiut University. Informed consent to participate in the study was obtained from participants. IRB:17100959, Date:10–02-2020.
Consent for publication
Applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shehata, K.M.A., Kelany, AA.I., Hassan, S.M.O. et al. Diagnostic accuracy of shear wave elastography versus laboratory parameters as non-invasive screening tool for esophageal varices. Egypt J Intern Med 36, 44 (2024). https://doi.org/10.1186/s43162-024-00311-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43162-024-00311-x