Abstract
Background
Several studies have suggested a causal link between acute kidney injury and the consequent development of chronic kidney disease. The severity, frequency, and duration of acute injury are key factors in this process.
Aims
This study aimed to determine the epidemiology and outcomes of AKI to CKD transition in critically ill adult patients and to study the role of Serpin-A3 in the early recognition of AKI to CKD transition.
Methods
In this prospective observational study, a total of 252 patients attending Assiut University Hospitals Critical Care Unit and developed AKI during their stay were recruited. Serum and urinary Serpin A3 were measured by ELISA Kit. Complete blood picture, kidney function tests, urine analysis, serum electrolytes (serum sodium, potassium, calcium, phosphorus, and magnesium), liver function test, coagulation profile, C-reactive protein, 24-h urinary protein or urinary albumin/creatinine ratio, abdominal ultrasound were assessed for all the recruited participants. Follow-up was done for three consecutive months and after 3 months using serum creatinine, BUN, and serum potassium.
Results
It was found that old age is a risk factor for CKD following AKI, i.e., with 1-year increase in the patient’s age, there was 3% increase in the chance of transition. Significant association was found between rate of comorbidity and transition status. Also, cases with either infection or IV radio contrast exposure were 2.8 and 6.5 times more liable for transition. Cases with transition in this study had significantly higher renal function parameters. Higher median levels of Serpin A3 either serum or urinary was reported in transition patients. Improvement was reported in two-third of those without transition, and higher mortality rate was recorded in those without transition.
Conclusion
The frequency of transition was 20%. Older age, male gender, cardiac and CVS disease, the presence of infection, higher BUN and creatinine level, higher median K and PO4 levels, and higher median levels of Serpin A3 are risk factors for transition from AKI to CKD.
Similar content being viewed by others
Introduction
Acute kidney injury (AKI) is a life-threatening and disabling complication of critical illness encountered about 30 to 60% of critically ill patients and is associated with acute morbidity and mortality [1]. AKI outcomes may vary from a complete resolution to a partial or incomplete recovery of renal function leading to increased mortality, prolonged hospitalization, and risk of chronic comorbidities like cardiovascular disease (CVD), chronic kidney disease (CKD), and subsequent progression to end-stage renal disease [2]. Several studies have suggested a causal link between AKI and the consequent development of CKD. The severity, frequency, and duration of AKI are key factors in this process [3].
The precise mechanism of transition from AKI to CKD is complex and not completely understood, especially in humans, and several pathways have been proposed [4,5,6,7]. Different animal studies have used ischemia–reperfusion and nephrotoxic injuries to investigate the pathophysiologic event involved in AKI to CKD transition, focusing on the development of specific histological changes [8].
The outcome of AKI depends upon the balance of adaptive and maladaptive repair. An adaptive response to injury usually leads to renal recovery with a complete resolution of pathological changes during AKI episode (resolution of inflammatory cell infiltration, regeneration of tubular cells, decrease in biomarkers of injury) without long-term consequences [9]. However, severe and repeated injury can result in a maladaptive repair, characterized by a permanent reduction in kidney function associated with significant structural changes [10].
Serpins are a family of serine protease inhibitors. Thirty-four serpins from 9 classes have been identified and characterized in humans to date; within these proteins is the serpinA3/alpha-1-antichymotrypsin (serine proteinase inhibitor, class A, member 3), whereas the homologous for rodents are serpinA3k [11]. SerpinA3 has been involved in different pathologies such as hypertension, inflammation, and angiogenesis [12]. However, little is known about the specific role of serpinA3 in the renal pathophysiology. One study showed increased serpinA3 staining in the proximal renal tubules in biopsies from a variety of primary and secondary glomerulonephritis, such as minimal-change disease (MCD), FSGS, diffuse mesangial proliferative glomerulonephritis, membranous glomerulonephritis (MGN), diabetic nephropathy, IgA nephritis, and LN compared to normal renal tissues [13]. As regard the expression of uSerpinA3 in human renal diseases. studies showed that uSerpinA3 is not detectable in healthy volunteers, but it increases in kidney diseases from different etiologies: LN, focal and segmental glomerulosclerosis (FSGS), and ANCA associated vasculitis (AAV). In LN patients, uSerpinA3 levels were found elevated in severe classes III/IV LN that are usually associated with greater kidney fibrosis, and less elevated, in the less inflammatory class V LN that usually carries better prognosis [14]. Studies showed that uSerpinA3 is an early and effective biomarker for the detection of renal injury, with a great potential to be used in the diagnosis of the AKI to CKD transition and CKD from different etiologies [15].
The current study aimed to determine the frequency, risk factors, and outcomes of transition of AKI to CKD in critically ill adult patients and to study the role of serpin-A3 in the early recognition of AKI to CKD transition.
Patients and methods
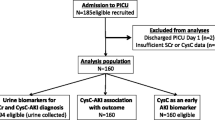
In this prospective observational study, a total of 252 patients attending Assiut University Hospitals Critical Care Unit and developed AKI, according to KDIGO criteria, were recruited.
Inclusion criteria were patients attending Assiut University Hospital’s Critical Care Unit and developing AKI during their stay. On the other hand, patients younger than 18 years or older than 80 years of age, preexisting CKD, ESRD prior ICU admission, and pregnancy were excluded.
Procedure
All patients were subjected to the following:
-
I.
Full history taking
-
II.
Thorough clinical examination
-
III.
The following investigations were carried out at admission: Complete blood picture, kidney function tests (KFT) (serum creatinine, blood urea nitrogen), urine analysis, serum electrolytes (serum sodium, potassium, calcium, phosphorus, and magnesium), liver function tests (ALT, AST, serum albumin), coagulation profile, C-reactive protein, 24-h urinary protein or urinary albumin/creatinine ratio according to availability in hospital, abdominal ultrasound (kidney size, length, echogenicity, cortical thickness, differentiation between cortex and medulla, obstruction, IVC collapsibility index, renal Doppler) CT chest, echocardiography (LV systolic function, diastolic dysfunction, valves morphology, pericardium).
Serum and urinary Serpin A3 which were done after the onset of AKI (was assessed by Human Serpin A3 ELISA Kit: Sandwich ELISA detection Sinogeneclon Biotech Co. Ltd. catalog no.: SG_13672).
Follow-up was done for three consecutive months and after 3 months using serum creatinine, BUN, and serum potassium.
Statistical analysis
IBM-SPSS 24.0 (IBM-SPSS Inc., Chicago, IL, USA) was used for data processing [16]. Means, standard deviations (SD), median, ranges, frequency, and percentages were calculated. Normality of continuous variables was tested using Kolmogorov–Smirnov test. Chi-square/Fisher’s exact test was used to compare the differences in distribution of frequencies among different groups. Student t-test/Mann–Whitney U analysis was carried out to compare the means/medians of dichotomous data. Paired sample t-test was carried out to compare the means of repeated data measurements. Multivariate logistic regression analysis was calculated to investigate the significant predictors of transition of AKI to CKD (odds ratio (OR), 95% confidence interval (95% CI), and p-value). ROC curve was depicted in the diagnostic performance of biomarkers for prediction of transition and analyzed as area under the curve (AUC), standard error (SE), and 95% CI. Validity statistics were calculated. Spearman ranked correlation was used to examine the univariate correlation between serpin levels and other laboratory parameters. A significant p-value was considered when it is > 0.05.
Ethical consideration
IRB approval was obtained from the Medical Ethical Committee, Faculty of Medicine, Assiut University (IRB no. 17101011). Trial registration was prospectively undertaken at ClinicalTrials.gov (NCT04101110). The study was carried out in accordance with the Helsinki Declaration guidelines [17] and in line with STROBE checklist for research ethics [18]. The title and objectives of the study were explained to them to ensure their cooperation. A written informed consent was obtained from the patient before the participation in the study. All collected data was confidential and was used for the purpose of scientific research only. Every research participant had the complete right and freedom to withdraw at any time from the study without any consequences on the medical service provided.
Results
This study was performed in the Critical Care Unit at Assiut University Hospitals. Two-hundred and fifty-two patients developing AKI during their stay at hospital were recruited to determine the epidemiology, risk factors, and outcomes of transition of AKI to CKD and followed up for 3 months. Incidence of transition from AKI to CKD among the studied population was 20.2%.
As shown in Table 1, patients with transition of AKI to CKD were significantly (p = 0.005) older (63.8 ± 13.9 years) than those without transition (56.3 ± 17.7 years). Moreover, there were significant difference in sex (p = 0.033). Higher rates of cardiac and CVS disease were reported in those with transition (57% and 22%) compared with those without (24.4% and 10%) (p = 0.001 and 0.024).
Nonsignificant levels of CBC parameters were recorded. For renal function test, cases with transition had higher median BUN (31 (12–87)) and serum creatinine (412 (187–1456) µmol/l) compared with non-transition group (26 (6–79)), and (412 (187–1456 µmol/l) (p = 0.024 and 0.002). Also, those with transition had higher median K level (4.5 (1–10) mmol/l) and PO4 level (4 (1–10) mg/dl) than non-transition group (4 (1–8) mmol/l) and (3 (1–13) mg/dl) (p = 0.049 and 0.002). In contrast, patients with transition had insignificant lower mean sodium, calcium, and magnesium than those without transition (p = 0.417, 0.123, and 0.598). Likewise, lower median total and direct bilirubin levels were observed in transition than non-transition patients (p = 0.532 and 0.392).
Notably, cases with transition had higher median levels of serpin either serum or urinary (169.5 (118–171) and 150 (114–282)) compared with non-transition group (74 (57.5–307) and 67 (40.5–147)) (p = 0.015 and 0.005). For pus cell number, patients with transition had significant (p = 0.001) higher median pus cell number (20 (5–100)) compared with non-transition group (12 (1–100)). Also, cases with transition had higher percentage of abnormal US and echo findings (n = 24 (47%) and n = 29 (57%)) compared with non-transition group (n = 79 (39%) and n = 67 (33%)), and this association was statistically significant (p = 0.048 and 0.002) (Table 2).
Table 3 showed the multivariable logistic regression model of the predictors of AKI transition to CKD among the studied group. It was found that with 1-year increase in the patient’s age, there was 3% (OR = 1.029, p = 0.010) increase in the chance of transition. The probability of transition was 4.25 times (OR = 4.246, p < 0.001) in cardiac cases. Likewise, the likelihood of transition was 2.3 times (OR = 2.331, p = 0.047) in patients with hepatic disease. Likely, cases with hematological disease were 8.25 times (OR = 8.246, p = 0.018) more liable for transition.
For risk factors, cases with either infection or IV radio contract were 2.8 and 6.5 times (OR = 2.792, p = 0.040 and OR = 6.549, p = 0.010) more liable for transition. For the laboratory data, 1 mg/dl increase in serum creatinine was correlated with increase in the chance of having transition by 0.2% (OR = 1.002, p = 0.003). Similarly, the increase of 1 mg/dl in the level of PO4 was associated with an increase in the chance of having transition by 17% (OR = 1.167, p = 0.035). Likewise, there was 7% (OR = 1.070, p = 0.014) rise in the possibility of transition with every point increase in the level of urinary serpin, and this was statistically significant. Additionally, the probability of transition showed 12% (OR = 1.012, p = 0.026) increase with every one-cell increase in RBCs count. As well, the likelihood of transition was 2.6 times (OR = 2.636, p = 0.002) in patients with abnormal US finding.
The mean BUN level showed significant (p < 0.001) reduction for both groups at follow-up than baseline reading. Also, cases with transition had significantly higher BUN level both at baseline and at 3-month follow-up compared with non-transition cases (p = 0.001 and 0.004). Moreover, the mean serum creatinine level showed significant (p < 0.001) reduction for both groups non-transition and transition at follow-up than baseline reading. Between groups, cases with transition had insignificantly higher serum creatinine level at baseline (p = 0.954), while at 3-month follow-up, it was significantly higher (p = 0.024). Furthermore, the mean potassium level showed significant (p < 0.001 and 0.047) reduction for both groups at follow-up than baseline reading. For between-group comparison, cases with transition had significantly higher mean potassium level both at baseline and at 3-month follow-up compared with non-transition cases (p = 0.049 and 0.001). Improvement of AKI was reported in two-third of those without transition (n = 134 (66.7%)) compared with transition group (0%) (p < 0.001). Likely, significantly (p = 0.003) higher mortality rate was recorded in those without transition (n = 66 (33%)) compared with transition group (n = 6 (12%)).
Table 4 showed the diagnostic value of the serum and urinary serpin level for prediction of transition. Both measures had excellent predictive power (AUC = 0.819 and 0.882, p = 0.017 and 0.004). For serum serpin and using a cut-off value of 117, the validity criteria were as follows: 100% sensitivity, 64% specificity, and the test had 73.5% precision-positive predictive value (PPV) and 100% negative predictive value (NPV). Overall, serum serpin had 82% accuracy. For urinary serpin and using a cut-off value of 113, sensitivity was 100%, specificity was 68%, and it had 74.5% PPV and 100% NPV with an overall 84% accuracy (Fig. 1).
Discussion
AKI is part of a variety of functional kidney conditions, which are summarized as acute kidney disease (AKD) and can range from mild and self-limiting to severe and persistent. AKD persisting for > 3 months is referred to as CKD [15]. The precise mechanism of transition from AKI to CKD is still unclear, especially in humans, and several pathways have been proposed [4,5,6,7].
This retrospective observational study included 252 AKI patients. The 3-month epidemiology of transition from AKI to CKD was 20.2%. The incidence in Sánchez Horrillo et al. study was higher, where after 3-month follow-up, 43% of AKI developed CKD [16]. Bucaloiu et al. suggested that 6.6% of patients with complete recovery of AKI exhibited a greater risk of death and de novo CKD after 2 to 4 years of follow-up [17]. In a retrospective study using data from 343 patients who survived an episode of dialysis-requiring AKI and 3430 matched patients without AKI, Lo and colleagues reported a 28-fold increased risk of developing advanced CKD following an episode of AKI [18].
Although observational studies have consistently identified an association between AKI and the development of CKD [19], uncertainty remains about whether this relationship is causal or whether it is confounded by the presence of other chronic conditions, which are themselves risk factors for AKI and CKD. Nonetheless, experimental models have demonstrated that AKI can result in chronic damage to the kidney parenchyma and thereby lead to CKD, suggesting that early intervention might influence long-term outcomes [20]. Renal parenchymal injury sustained during episodes of AKI may lead to permanent tubulointerstitial fibrosis and a reduction in the number of functioning nephrons [21].
Among demographic variables, old age is a risk factor for CKD following AKI. In the present study, patients with transition were significantly older. It was found that with a 1-year increase in the patient’s age, there was 3% increase in the chance of transition. Similarly, in Sánchez Horrillo et al. study, the AKI-to-CKD transition was more frequent in older patients [22]. Several studies agreed with the current study, and an age was associated with progression to CKD [17, 23, 24].
An association was found between rate of comorbidity and transition status, where higher rates of cardiac and CVS disease were reported in those with transition. Supporting the present study, Bucaloiu and colleagues detected that a history of congestive heart failure was significant predictor of subsequent CKD after AKI. However, contrasting to this study, they found that hypertension can predict occurrence of CKD after AKI [17]. Contradicting this study, Chawla et al. reported that having DM added 24% risk of CKD on top of AKI [23]. In Sánchez Horrillo et al. study, the AKI-to-CKD transition was more frequent in patients with comorbidities [22]. Also, Pereira and coworkers found that the progression of AKI to CKD was more frequent in cancer patients (74.19%) [25]. Regarding the risk factors, cases with either infection or IV radio contract exposure were 2.8 and 6.5 times more liable for transition. This can be explained by the fact that infections contribute to the development and progression of CKD and complicate the course of patients with preexisting CKD [26].
Cases with transition in this study had higher BUN level both at baseline and at 3-month follow-up. Likewise, the median level of serum creatinine was higher in the transition group. Cases with transition had insignificantly higher serum creatinine level at baseline, while at 3-month follow-up it was significantly higher. The same was reported in Li et al. who found that patients with non-recovery (CKD) had higher creatinine (137.6 µmol/l vs 125.0 µmol/l, p < 0.001) and peak creatinine (173.3 µmol/l vs 129.8 µmol/l, p < 0.001) levels, as well as a higher BUN (17.0 mmol/l vs 11.0 mmol/l, p < 0.001) level compared with patients with renal recovery after AKI diagnosis [27]. Also, Chawla et al. detected that each mg/dl unit increase for SC during hospitalization added 50% to the odds of reaching CKD4 [23].
The current study found that cases with transition had significantly higher median K and PO4 levels. Patients with transition had lower mean sodium, calcium, and magnesium than those without transition. The mean potassium level showed significant reduction for both groups non-transition and transition at follow-up than baseline reading. Supporting this study, Chen et al. study found that abnormal serum sodium or potassium levels before AKI diagnosis were more likely to lead to AKI progression and poor prognosis [28].
Notably, cases with transition had higher median levels of serpin either serum or urinary. This agreed with Sánchez-Navarro et al. study which was conducted on a rat model, and their study supports that uSerpinA3 is an early and effective biomarker for the detection of renal injury, with a great potential to be used in the diagnosis of the AKI to CKD transition and CKD from different etiologies. This can be explained as follows: in pathologic conditions, serpinA3 was relocated from the cytoplasm to the apical epithelial membrane. This suggests that uSerpinA3 reflects intrarenal injury; during renal injury, it is probably secreted into the luminal space explaining its emergence in the urine, and uSerpinA3 is an early and timely marker of transition [16].
Additionally, higher % of patients with positive cast, albumin, and 24-h protein were reported in transition cases than those without transition, and this was statistically insignificant. Conversely, James and colleagues detected that albuminuria was a risk factors for CKD following AKI [29]. Cases with transition had a significantly higher percentage of abnormal US and echo findings compared with non-transition group. Unlikely, the percentage of abnormal chest CT was insignificantly higher in the transition group. This agreed with Katagiri et al. conclusion [30].
Improvement of AKI was reported in two-third of those without transition. Likely, higher mortality rate was recorded in those without transition. Bucaloiu et al. found that despite near-equivalent death rates for those matched patients with and without AKI who do not subsequently develop CKD, the more-than-threefold increased risk of death for those matched patients with AKI who developed CKD relative to the non-AKI controls who did not develop CKD is striking [17]. The observation that the mortality risk associated with AKI in this population is largely attenuated by the development of CKD is consistent with a large body of literature linking CKD itself with an increased risk of death, relative to those with normal kidney function [19]. An association between AKI and long-term mortality has previously been observed among patients with established CKD or in mixed populations with and without CKD. This relationship appears to be consistent both among those with more severe, dialysis-requiring AKI and those with less severe cases of AKI [30]. This finding would suggest that any long-term mortality risk among patients with recovered AKI is largely influenced by the subsequent development of CKD rather than the AKI event itself. We suspect that the difference in definition of recovery explains the discrepancy in mortality risk between this study and the results we report.
Conclusion
In conclusion, the 3-month frequency of transition from AKI to CKD among the adult patients attending Assiut University Hospitals Critical Care Unit was 20.2%. Older age, male gender, cardiac and CVS disease, the presence of infection, higher BUN and creatinine level at 3-month follow up, higher median K and PO4 levels, and higher median levels of Serpin A3 are risk factors for transition from AKI to CKD. U/S can be used as a predictor and diagnostic for transition from AKI to CKD.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Hoste E, Kellum J, Selby N, Zarbock A, Palevsky P, Bagshaw S, Goldstein S, Cerdá J, Chawla L (2018) Global epidemiology and outcomes of acute kidney injury. Nat Rev Nephrol 14(10):607–625
Goldstein SL, Chawla L, Ronco C, Kellum J (2014) Renal recovery. Crit Care 18:301
Heung M, Steffick D, Zivin K, Gillespie B, Banerjee T, Hsu C, Powe N, Pavkov M, Williams D, Saran R, Shahinian V, Centers for Disease Control and Prevention CKD Surveillance Team (2016) Acute kidney injury recovery pattern and subsequent risk of CKD: an analysis of Veterans Health Administration data. Am J Kidney Dis. 67(5):742–52
Makris K, Spanou L (2016) Acute kidney injury: definition, pathophysiology and clinical phenotypes. Clin Biochem Rev 37(2):85–98
Lee S, Cozzi M, Bush E, Rabb H (2018) Distant organ dysfunction in acute kidney injury: a review. Am J Kidney Dis 72(6):846–856
Zhu Z, Hu J, Chen Z, Feng J, Yang X, Liang W, Ding G (2022) Transition of acute kidney injury to chronic kidney disease: role of metabolic reprogramming. Metabolism 131:155194
Venkatachalam M, Weinberg J, Kriz W, Bidani A (2015) Failed tubule recovery, AKI-CKD transition, and kidney disease progression. J Am Soc Nephrol 26(8):1765–1776
Basile D, Bonventre J, Mehta R, Nangaku M, Unwin R, Rosner M, Kellum J, Ronco C, ADQI XIII Work Group (2016) Progression after AKI: understanding maladaptive repair processes to predict and identify therapeutic treatments. J Am Soc Nephrol 27(3):687–97
Chou Y, Huang T, Chu T (2017) Novel insights into acute kidney injury-chronic kidney disease continuum and the role of renin-angiotensin system. J Formos Med Assoc 116(9):652–659
Chou Y, Huang T, Pan S, Chang C, Lai C, Wu V, Wu M, Wu K, Chu T, Lin S (2017) Renin-angiotensin system inhibitor is associated with lower risk of ensuing chronic kidney disease after functional recovery from acute kidney injury. Sci Rep 7:46518
Van Gent D, Sharp P, Morgan K, Kalsheker N (2003) Serpins: structure, function and molecular evolution. Int J Biochem Cell Biol 35(11):1536–1547
Liu X, Lin Z, Zhou T, Zong R, He H, Liu Z et al (2011) Antiangiogenic and anti-inflammatory effects of SERPINA3K on corneal injury. PLoS One 6(1):e16712
Conz P, Bevilacqua PA, Ronco C, Feriani M, Brendolan A, Dell’Aquila R et al (1990) Alpha-1-antichymotrypsin in renal biopsies. Nephron 56(4):387–390
Sun HO, Hu WX, Xie HL, Zhang HT, Chen HP, Zeng CH et al (2008) Long-term outcome of Chinese patients with membranous lupus nephropathy. Lupus 17(1):56–61
Sánchez-Navarro A, Mejía-Vilet JM, Pérez-Villalva R, Carrillo-Pérez DL, Marquina-Castillo B, Gamba G et al (2019) SerpinA3 in the early recognition of acute kidney injury to chronic kidney disease (CKD) transition in the rat and its potentiality in the recognition of patients with CKD. Sci Rep 9(1):10350
.IBM_SPSS. Statistical Package for Social Science. Ver.24. Standard version. Copyright © SPSS Inc., 2012–2016. NY, USA. 2016.
World Medical Association (2013) World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 310(20):2191–2194
Gallo V, Egger M, McCormack V et al (2012) the Reporting of Observational studies in Epidemiology - Molecular Epidemiology (STROBE-ME): an extension of the STROBE statement. Eur J Clin Invest 42(1):1–16
Khwaja A (2012) KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 120(4):c179–c184
Sánchez-Navarro A, Mejía-Vilet J, Pérez-Villalva R, Carrillo-Pérez D, Marquina-Castillo B, Gamba G (2019) SerpinA3 in the early recognition of acute kidney injury to chronic kidney disease (CKD) transition in the rat and its potentiality in the recognition of patients with CKD. Sci Rep 9(1):10350
Bucaloiu I, Kirchner H, Norfolk E, Hartle J, Perkins R (2012) Increased risk of death and de novo chronic kidney disease following reversible acute kidney injury. Kidney Int 81(5):477–485
Lo L, Go A, Chertow G, McCulloch C, Fan D, Ordoñez J et al (2009) Dialysis-requiring acute renal failure increases the risk of progressive chronic kidney disease. Kidney Int 76(8):893–899
See E, Jayasinghe K, Glassford N, Bailey M, Johnson D, Polkinghorne K (2019) Long-term risk of adverse outcomes after acute kidney injury: a systematic review and meta-analysis of cohort studies using consensus definitions of exposure. Kidney Int 95(1):160–172
Ftouh S, Thomas M (2013) Acute kidney injury: summary of NICE guidance. BMJ 347:f4930
Spurgeon-Pechman K, Donohoe D, Mattson D, Lund H, James L, Basile D (2007) Recovery from acute renal failure predisposes hypertension and secondary renal disease in response to elevated sodium. Am J Physiol Physiol 293(1):F269–F278
Sánchez Horrillo A, Villanueva L, Cárdenas A, Ramos P, Ortiz A, Quiroga B (2022) Infectious consequences of the AKI-to-CKD transition. Clin Kidney J 15(12):2237–2244
Chawla L, Eggers P, Star R, Kimmel P (2014) Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med 371(1):58–66
Lameire N, Bagga A, Cruz D, De Maeseneer J, Endre Z, Kellum J (2013) Acute kidney injury: an increasing global concern. Lancet 382(9887):170–179
Pereira B, Barreto S, Gentil T, Assis L, Soeiro E, Castro I (2017) Risk factors for the progression of chronic kidney disease after acute kidney injury. Brazilian J Nephrol 39:239–245
Jha V, Prasad N (2016) CKD and infectious diseases in Asia Pacific: challenges and opportunities. Am J Kidney Dis 68(1):148–160
Acknowledgements
Not applicable
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
AAG, concept, design, literature search, clinical studies, statistical analysis, manuscript preparation, editing, and review. MHM, design, literature search, manuscript preparation, and review. MAD and SSP, clinical and laboratory work. MKK, literature search, clinical studies, manuscript editing, and final draft. MHM, manuscript preparation, editing, review, and final draft for journal submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted according to the principles of the Declaration of Helsinki and was approved by the hospital’s ethics committee, and purpose of the study was explained to all participants, and written informed consent was obtained. The study was approved by Assiut Faculty of Medicine, Institutional Review Board (IRB No. 17101011, 2021). The study was explained to all patients, and only patients who were signing an informed consent were participated in study. This study was registered on ClinicalTrials.gov with identifier NCT04101110.
Consent for publication
Consent was taken from participants for publication.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khairallah, M.K., Gaber, A.A., Maghraby, M.H. et al. Epidemiology, risk factors, outcomes, and role of Serpin A3 as a biomarker for transition of acute kidney injury to chronic kidney disease in critically ill patients. Egypt J Intern Med 36, 30 (2024). https://doi.org/10.1186/s43162-024-00291-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43162-024-00291-y