Abstract
Background
Interleukin (IL)-36α and IL-38, two novel cytokines of the IL-1 family, have recently been proposed to have a pathophysiological significance in type 2 diabetes mellitus (T2DM). However, there is a paucity of information regarding their association with diabetic neuropathy (DNP). Therefore, this study aimed to explore these interleukins in T2DM without and with DNP, referred to as T2D and DNP, respectively. The predicted interaction of IL-36α and IL-38 with other proteins was also analyzed bioinformatically. In this study, 85 T2D patients, 21 DNP patients, and 109 controls were recruited. Serum IL-36α and IL-38 concentrations were measured with ELISA kits.
Results
Median (interquartile range) of IL-36α concentrations was significantly greater in T2D and DNP patients compared with controls (62 [54-84] and 52 [45-56] vs. 44 [36-47] pg/mL, respectively; p < 0.001). T2D patients also exhibited significantly greater concentrations of IL-36α than DNP patients (p = 0.004). IL-38 concentrations were significantly greater in T2D and DNP patients compared with controls (208 [149-249] and 200 [130-253] vs. 64 [47-92] pg/mL, respectively; p < 0.001), while T2D and DNP patients showed no significant differences in IL-38 concentrations (p = 0.509). Both cytokines were reliable biomarkers in differentiating diabetic patients from controls, but differentiation performance was better in T2D (area under the curve [AUC] = 0.921 and 0.951, respectively) than in DNP (AUC = 0.881 and 0.844, respectively). Up-regulated IL-36α and IL-38 concentrations were significantly associated with a higher risk of T2D (37.92- and 29.97-fold, respectively) and DNP (10.11- and 32.47-fold, respectively). IL-36α was positively correlated with IL-38 in T2D (correlation coefficient [rs] = 0.487; p < 0.001), but a stronger correlation was found in DNP (rs = 0.683; p < 0.001). IL-36α and IL-38 showed predicted interactions with several cytokines and cytokine receptors of the IL-1 family.
Conclusions
IL-36α and IL-38 concentrations were upregulated in the serum of T2D and DNP patients. Both cytokines were indicated to be potential discriminating biomarkers associated with higher risk of T2D and DNP. Targeting the axis of their interaction with other cytokines of the IL-1 family may be important for understanding the pathophysiology of T2D and DNP.
Similar content being viewed by others
Background
Type 2 diabetes mellitus (T2DM) is the most common metabolic disorder among adults accounting for approximately 95% of all diabetic cases in the global population with a significant impact on healthcare standards and disease management programs [1]. The disease is associated with the progression of several comorbidities, such as neuropathy and retinopathy, which further complicate the control of T2DM [2]. T2DM is characterized by hyperglycemia due to insulin secretion defects and an appropriate cellular response to insulin [3]. The pathophysiology of T2DM is complex, and the underlying mechanism is not well deciphered. However, several interacting factors, including genetic predisposition, obesity, lifestyle, and others, have been identified to have an etiological role in the disease [4]. In addition, the pathogenesis of T2DM and its complications have been associated with dysregulated immune functions, particularly those involving pro- and anti-inflammatory cytokines [5, 6].
Cytokines are the key signaling messengers involved in controlling functional communications and in mediating inflammatory reactions [7]. There is compelling evidence to suggest that pro-inflammatory mediators, such as tumor necrosis factor (TNF)-α, macrophage chemotactic protein-1, interleukin (IL)-1β, and IL-6, are involved in insulin resistance and pancreatic β-cell apoptosis [8]. In addition, anti-inflammatory cytokines, such as adiponectin and transforming growth factor-β1 (TGF-β1), have also been associated with the pathogenesis of T2DM, and anti-inflammatory therapies are suggested to improve glycemic control in T2DM patients [9, 10]. Both pro- and anti-inflammatory cytokines have been indicated to be dysregulated in T2DM and have been proposed to be associated with disease risk. In fact, it is increasingly recognized that T2DM is linked low-grade systemic inflammation [6, 11].
IL-36 and IL-38, two novel cytokines of the IL-1family, are among the cytokines proposed to exert a functional role in regulating the homeostasis of the immune system as well as being involved in mediating the pathogenesis of inflammatory diseases [8]. Regarding T2DM, recent studies have pointed out that both cytokines are connected to the pathophysiology of the disease, although the evidence is limited as few studies have been conducted [12,13,14,15]. Besides, their pathological role in diabetic neuropathy (DNP) has not been well investigated [16].
IL-36, a cytokine with pro-inflammatory functions, is recognized to have three agonists (α, β, and γ) and a natural antagonist (IL‐36Ra), which participate in a functional regulation of immune and inflammatory responses [17]. Recently, IL-36 cytokines have been related to low-grade inflammation, insulin resistance, and obesity, and thus, their role in T2DM risk and pathogenesis has been proposed [12, 13]. Regarding IL-38, it is an anti-inflammatory cytokine that has been indicated to show dysregulated levels in a number of inflammatory and autoimmune conditions. Accordingly, IL-38 role in the pathogenesis of these conditions has been disclosed, and its potential as therapeutic target has been proposed [18, 19]. In the setting of diabetes, two recent studies have provided evidence of dysregulated production of IL-38 in gestational diabetes and T2DM [14, 20].
In the current research, serum IL-36α and IL-38 concentrations were examined in T2DM patients with a focus on those who developed DNP as no studies have been performed in this regard. Furthermore, the significance of IL-36α and IL-38 as discriminating serological markers between T2DM without DNP (referred to as T2D) and HC or T2DM with DNP (referred to as DNP) and HC was evaluated. The risk association of IL-36α and IL-38 with T2D and DNP was also analyzed. Furthermore, bioinformatics analysis was conducted to predict the interactions of L-36α and IL-38 with other immune proteins.
Methods
Populations studied
A case-control study was conducted on 85 patients with T2D (T2DM without DNP; 49.4% males and 50.6% females), 21 patients with DNP (T2DM with DNP; 47.6% males and 52.4% females), and 109 healthy controls (HC; 49.5% males and 50.5% females) during the period November 15, 2021-February 10, 2022. The median age (interquartile range; IQR: 25–75%) in the T2D, DNP, and HC groups was 52 (47–60), 59 (52–67), and 52 (46–56) years, respectively. Patients were registered and diagnosed at the National Center for Diabetes (University of Mustansiriyah, Baghdad). DNP (diabetic sensorimotor neuropathy) was determined using the Toronto Clinical Neuropathy Scoring-System (TCNS). The TCNS score ranges from 0 to 19, and patients can be classified as having no neuropathy or having mild, moderate, or severe neuropathy (scores: 0–5, 6–8, 9–11, and ≥ 12, respectively) [21]. The TCNS score of the current 21 DNP patients was 6–8, and they were therefore classified as having mild neuropathy. All patients were taking oral hypoglycemic medications. Only patients aged 18 years and older with adult-onset disease were included. Excluded patients were those with T1DM, gestational diabetes, diabetes comorbidities other than DNP, cardiovascular complications, or cancer. Data pertaining to patients (age, gender, onset age, and duration of disease) were obtained from patient records. Body mass index (BMI) and waist-to-hip ratio (WHR) were also determined. Baseline laboratory tests included FPG (fasting plasma glucose), HbA1c (glycated hemoglobin), lipid profile, and liver function tests. The HC group included blood donor volunteers and hospital staff who did not suffer from diabetes or other chronic and infectious diseases, and their FPG (79 [IQR: 76–84] mg/dL) and HbA1c (5.4 [IQR: 5.4–5.8] %) were within the reference ranges [22].
Measurement of BMI and WHP
A weight-height scale machine (Zhengzhou Technology, China) was used to measure the weight and height of T2D and DNP patients. For body weight, it was adjusted to the closest 0.1 kg, while for body height, it was adjusted to the closest 0.1 m. BMI was calculated in kg/m2 (weight divided by height squared). In the case of waist and hip circumferences, a tape measure was used, and the measurement was adjusted to the nearest 0.1 cm. To determine WHR, the waist measurement was divided by the hip measurement [23].
Laboratory methods
Morning blood was collected in EDTA tubes and plain vacutainer tubes after fasting for 10–12 h. EDTA blood was used in the assessment of FPG and HbA1c. Plain tube blood was centrifuged for 10 min at 1200 × g to separate serum, which was frozen (−20 °C) until assessment of lipid profile, liver function, and cytokines. An automated biochemical analyzer (Cobas c311, Roche, Germany) was used to measure FPG, HbA1c, lipid profile, and liver function parameters. Serum concentrations of IL-36α and IL-38 were measured with commercial ELISA kits provided by MyBioSource (USA), and the manufacturer’s protocol was followed.
Bioinformatics analysis
Bioinformatics analysis was conducted to predict IL-36α and IL-38 interacting proteins using the STRING database (https://string-db.org). Only protein-protein interactions with a score > 0.75 were taken into account.
Statistical analysis
The Shapiro-Wilk normality test indicated that the continuous variables followed a nonparametric distribution and were therefore expressed with the median and IQR. Statistically significant differences between medians were evaluated using the Mann-Whitney U-test. Receiver operating characteristic (ROC) curve analysis was applied to evaluate the potential of IL-36α and IL-38 as biomarkers to differentiate T2D and DNP patients from HC. In light of this, the area under the curve (AUC) and 95% confidence interval (CI) were calculated. To analyze the association with T2D and DNP risk, the odds ratio (OR) and 95% CI were estimated after categorizing serum IL-36α and IL-38 concentrations into two groups, low-production group (LP; ≤ median), and high-production group (HP; > median), and then HP versus LP analysis was performed. Spearman's rank-order correlation test was applied to analyze the pairwise correlation between variables. Results were presented in the form of a heat-map matrix of the correlation coefficient (rs). A probability (p) < 0.05 was chosen to indicate statistical significance. GraphPad Prism (San Diego, CA, USA) was used to accomplish statistical analysis. G*Power software was used to compute the sample size and to determine the sample size power [24].
Results
Sample size calculation and power analysis
An a priori analysis was conducted to estimate the required sample size for diabetic patients and HC at 0.05 two-tailed α error p, 0.5 effect size d, and 0.95 power (1-β error p) using G*Power software. The analysis revealed that a minimum of 105 participants in each group achieves a statistically acceptable power of sample size. In light of this, the study included 106 patients with T2DM (85 T2D and 21 DNP) and 109 HC.
Baseline characteristics
A comparison of T2D patients with DNP patients was made with respect to the clinical, demographic, and laboratory data presented in Table 1. Only two significant differences were observed. Age (59 [IQR: 52–67) vs. 52 [IQR: 47–60) years; p = 0.04) and disease duration (10 [IQR: 7–15] vs. 6 [IQR: 3–10] years; p = 0.05) were higher in DNP than in T2D. Of note, glycemia was poorly controlled in both groups of patients as indicated by HbA1c > 8%. In addition, T2D and DNP patients were on the threshold for obesity as indicated by a BMI > 30 kg/m2 and a WHR > 0.90 [23]. Liver function and lipid profile parameters were approximately within the reference ranges and showed no significant differences between T2D and DNP patients.
IL-36α and IL-38 concentrations
IL-36α concentrations were significantly greater in T2D patients and DNP patients compared to HC (62 [IQR: 54–84] and 52 [IQR: 45–56] vs. 44 [IQR: 36–47] pg/mL; p < 0.001). IL-36α concentrations were also significantly greater in T2D patients compared to DNP patients (p = 0.004). IL-38 concentrations were significantly increased in T2D and DNP patients compared to HC (208 [IQR: 149–249] and 200 [IQR: 130–253] vs. 64 [IQR: 47–92] pg/mL; p < 0.001), while the differences were not significant between T2D and DNP patients (p = 0.509) (Fig. 1). IL-36α and IL-38 were excellent in differentiating diabetic patients from controls, but differentiation performance was better in T2D (AUC = 0.921 and 0.951, respectively) than in DNP (AUC = 0.881 and 0.844, respectively) (Fig. 2). Association analysis revealed that individuals with high IL-36α concentrations were at significantly higher risk of T2D (OR = 37.92; 95% CI = 16.59–86.67; p < 0.001). In DNP, there was also a significant increase in risk associated with elevated IL-36α concentrations, but the OR was lower (OR = 10.11; 95% CI = 3.65–28.00; p < 0.001). Regarding IL-38, higher risk of both T2D and DNP was significantly associated with increased concentrations of this cytokine, but the odds ratio was higher in DNP (OR = 32.47; 95% CI = 8.86–119.05; p < 0.001) compared to T2D (OR = 29.97; 95% CI = 13.73–65.45; p < 0.001) (Table 2).
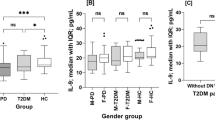
Box-whisker plots of IL-36α (plot A) and IL-38 (plot B) levels in the serum of type 2 diabetes mellitus patients without diabetic neuropathy (T2D; n = 85), type 2 diabetes mellitus patients with diabetic neuropathy (DNP; n = 21), and healthy controls (HC; n = 109). The horizontal line inside boxes indicates median. Whiskers indicate interquartile range (IQR: 25–75%). Significance was assessed using Mann-Whitney U-test (**p < 0.01; ***p < 0.001; ns: not significant)
Receiver operating characteristic (ROC) curve analysis of IL-36α (plots A and B) and IL-38 (plots C and D) in type 2 diabetes mellitus patients without diabetic neuropathy (T2D; n = 85) and type 2 diabetes mellitus patients with diabetic neuropathy (DNP; n = 21). Area under the curve (AUC) and two-tailed probability (p) are shown. IL-36α and IL-38 were excellent in differentiating diabetic patients from controls, but differentiation performance was better in T2D (AUC = 0.921 and 0.951, respectively) than in DNP (AUC = 0.881 and 0.844, respectively)
Correlation analysis
Spearman’s rank-order correlation analysis was performed between IL-36α, IL-38, BMI, WHR, FPG, and HbA1c. In T2D and DNP, IL-36α showed a positive correlation with IL-38 (rs = 0.487; p < 0.001 and rs = 0.683; p < 0.001, respectively) and a negative correlation with WHP (rs = −0.215; p < 0.05 and rs = −0.447, p < 0.05). In DNP, IL-38 also showed a negative correlation with WHP (rs = −0.539, p < 0.01). FPG and HbA1c showed a positive correlation in both T2D and DNP. HbA1c was also positively correlated with BMI in DNP (rs = 0.460; p < 0.05) (Fig. 3).
Heat-map matrix of Spearman’s rank-order correlation analysis between IL-36α, IL-38, body mass index (BMI), waist-to-hip ratio (WHR), fasting plasma glucose (FPG), and glycated hemoglobin (HbA1c) in type 2 diabetes mellitus patients without diabetic neuropathy (T2D; plot A) and type 2 diabetes mellitus patients with diabetic neuropathy (DNP; plot B). Values inside boxes indicate the correlation coefficient. Significant correlation (two-tailed probability; p) is indicated by an asterisk (*p < 0.05; **p < 0.01; ***p < 0.001). Blue indicates a positive correlation. Red indicates a negative correlation
Bioinformatics analysis
Bioinformatics analysis predicted that IL-36α interacts with five proteins arranged in a descending manner according to their interaction scores: IL-1 receptor-like 2 (IL1RL2; 0.996), IL-1 receptor accessory protein (IL1RAP; 0.908), IL-36 receptor antagonist protein (IL36RN; 0.789), IL-1α (IL1A; 0.789), and IL-36β (IL36B; 0.78). IL-38 (IL1F10) also interacts with IL1RL2 (0.999), IL-1 receptor accessory protein-like-1 (IL1RAPL1; 0.957), IL-36β (0.868), and the IL-1 receptor type 1 (IL1R1; 0.898) and karyopherin subunit alpha 7 (KPNA7; 0.838) (Fig. 4).
Prediction of the protein-protein interaction network IL-36α (A IL36A) and IL-38 (B IL1F10) with relevant proteins (interaction score > 0.75) as revealed by STRING analysis (https://string-db.org). Colored nodes indicate IL-36α, IL-38, and first shell of interactions. Edges indicate IL-36α- and IL-38-protein association. Connections were identified as colored lines. IL1A, IL-1α; IL1RL2, IL-1 receptor-like 2; IL1RAP, IL-1 receptor accessory protein; IL1RAPL1, IL-1 receptor accessory protein-like-1; IL36RN, IL-36 receptor antagonist protein; IL1R1, IL-1 receptor type 1; KPNA7, karyopherin subunit alpha 7
Discussion
In the current study, two diabetic groups were enrolled: T2D (T2DM patients without DNP) and DNP (T2DM patients with DNP). Notably, DNP was associated with a higher age and longer disease duration than T2D. DNP is among the most prevalent diabetes-associated comorbidities occurring in approximately 50% of patients. Age and duration of disease may have predisposing effects along with uncontrolled hyperglycemia [2]. Indeed, most of the current diabetic patients had poorly controlled hyperglycemia that was evident by FPG and HbA1c levels of 180 mg/dL and > 8.0%, respectively, which significantly exceeded the threshold of the two tests as described in the ADA guidelines; ≥ 126 mg/dL for FPG and ≥ 6.5% for HbA1c [22]. In addition, BMI and WHR classified current diabetic patients as obese. Accumulating evidence indicates that obesity is the most important risk factor linked to diabetes and associated comorbidities, and glycemic control has been shown to improve significantly with a weight loss of 5% [25]. Moreover, epidemiological surveys disclosed that the increasing prevalence of obesity worldwide has a significant impact in increasing the prevalence of T2DM and associated comorbidities [26].
The primary focus of this study was to understand the pathological significance of IL-36α and IL-38 in T2D and DNP and highlight their role in the risk of developing both conditions because the data available in this context are not overwhelming especially for DNP. The results demonstrated that IL-36α and IL-38 might have a contributing functional role in mediating the pathogenesis of T2D and DNP. Both cytokines showed elevated levels in T2D and DNP patients compared with HC. The up-regulated concentrations of IL-36α and IL-38 were excellent in distinguishing T2D patients from HC (AUC = 0.921 and 0.951, respectively), as well as DNP patients from HC (AUC = 0.811 and 0.884, respectively). Besides, elevated IL-36α and IL-38 concentrations were linked to a 37.92- and 29.97-fold increased risk of T2D, respectively, and a 10.11- and 32.47-fold increased risk of DNP. Based on these findings, IL-36α and IL-38 can be considered important cytokines of the IL-1 family associated with the risk of T2D and DNP. Both cytokines may also be considered as a potential therapeutic target in T2DM and its clinical complications such as DNP.
IL-36 cytokines have recently been recognized for their role in maintaining immune hemostasis and regulating the functions of the immune system through pro-inflammatory effects [27]. Therefore, the three IL-36 isoforms (α, β, and γ) and the antagonist IL-36Ra have sparked significant interest to determine their potential in the pathogenesis of a number of inflammatory and autoimmune diseases, for instance, systemic lupus erythematosus, inflammatory bowel disease, and rheumatoid arthritis. Up-regulated expressions and elevated levels of one or more of these isoforms have been found, while IL-36Ra has been indicated to be downregulated [28,29,30]. This imbalance favors a pro-inflammatory state because IL-36 cytokines (α, β, and γ) are involved in the activation of IL-36R signaling, whereas the opposite effect can be induced by IL-36Ra [27]. The current study evaluated one of the IL-36 isoforms, IL-36α, in T2D and DNP patients and found significantly elevated serum levels compared to nondiabetic subjects (HC group). Three recent study reported consistent findings. In the first, IL-36α and IL-36γ concentrations were elevated in T2DM patients. In addition, IL-36α exhibited a positive correlation with the inflammatory marker high-sensitivity C-reactive protein and a negative correlation with IL-36Ra [13]. A second study demonstrated that IL-36γ was linked to obesity and T2DM, and its role in regulating adipose tissue hemostasis and chronic inflammation was suggested [12]. A third study linked urine IL-36α levels to DNP and FPG in T2DM patients [16]. The present study results are consistent with these studies and suggest that IL-36α is associated with the risk of T2DM and may have a prognostic value. Additionally, elevated concentrations of IL-36α may also contribute to an increased risk of DNP.
Besides IL-36α, IL-38 is a further cytokine of the IL-1 family that showed increased concentrations in T2D and DNP patients. In line with these findings, children with a recently diagnosed T2DM showed increased serum IL-38 levels, particularly those who were sensitive to insulin therapy. Sensitivity to insulin was examined in more detail in a mouse model of T2DM, and IL-38 was proposed to attenuate the progression of T2DM by increasing sensitivity to insulin. This effect could also be achieved by suppressing the pro-inflammatory effects of IL-36 in these mice, as anti-IL-36 antibody reduced the plasma insulin level and increased insulin sensitivity [15]. A further study also showed that plasma IL-38 levels were significantly increased in elderly T2DM patients (mean age ± SD = 67.6 ± 7.1 years), particularly those with DNP, and were positively correlated with HbA1c, triglycerides, and liver function tests, as well as WHP [14]. Our study confirms the elevated levels of IL-38 in T2DM and DNP, but IL-38 was negatively correlated with WHP in DNP patients. This difference in the correlation pattern is likely related to the difference in the age of the included patients because Gurău’s study included only elderly patients [14]. In contrast to these findings, T2DM patients with and without latent tuberculosis showed lower IL-38 levels compared with HC, whereas patients with only latent tuberculosis showed elevated levels [31]. However, the association between IL-38 and diabetes was further confirmed in patients with gestational diabetes. Immunohistochemical examination of the placenta in these patients showed that IL-38 expression was significantly increased in the chorionic villi, umbilical artery, and umbilical vein by 3.3-, 2.6-, and 2.6-fold, respectively. Accordingly, the authors suggested that this increased expression represents an anti-inflammatory response in the placenta environment during the development of gestational diabetes [32].
Correlation analysis revealed that IL-36α and IL-38 were positively correlated in T2D and DNP, but the correlation was stronger in DNP than in T2D (rs: 0.683 vs. o.487). Although there is no confirming evidence, this may underline their functional relationships in terms of dynamic regulation and cellular interactions. The initiation and progression of T2DM are associated with multiple risk factors, including age, obesity, diet, genetic predisposition, and others, which impact both immune homeostasis and inflammatory processes [5]. In this context, cytokines are the major players in orchestrating the inflammatory cascade through pro- and anti-inflammatory functions. The imbalance between the two functions may lead to T2DM progression [33]. The current study included cytokines that have pro-inflammatory (IL-36α) and anti-inflammatory (IL-38) effects. Both cytokines showed up-regulated serum levels and were positively correlated. This correlation patterns in general may indicate that elevated levels of anti-inflammatory cytokines occur in response to the pro-inflammatory environment to downregulate the exacerbated inflammatory process and maintain physiological homeostasis in vital organs [34]. Furthermore, bioinformatic analysis predicted that IL-36α and IL-38 interact with members of the IL-1 family of cytokines and cytokine receptors, such as IL1RL2, IL1RAP, IL36RN, IL1A, IL36B, and IL1R1, which have been implicated in inflammatory signaling pathways in several inflammatory diseases including T2DM [13, 35]. These data may suggest a functional interaction between these cytokines and cytokine receptors in the pathogenesis of T2DM. Interestingly, IL-38 showed an additional interaction, which was with KPNA7, a gene encoding a protein belonging to the importin alpha family that facilitates the translocation of signal-containing proteins into the nucleus [36]. Importin expression was revealed to be an important regulator of the NF-κB (nuclear factor kappa B) transcription factor signaling pathway [37]. This pathway is involved in the control of inflammation and immunity and may play a crucial role in the pathogenesis of diseases due to dysregulated inflammatory and immune responses such as cardiovascular diseases [38]. On the other hand, the NF-κB signaling pathway can be activated by persistent hyperglycemia, and as a result, the production of various cytokines and inflammatory molecules involved in the pathogenesis of T2DM is dysregulated [39]. Therefore, understanding the IL-38-NF-κB-KPNA7 interaction axis may represent an important strategy for research aimed at unraveling the pathogenesis of T2DM, and investigations in this context are certainly encouraged.
The study encountered the following limitations. The number of DNP patients included was small. Homeostasis model assessment (HOMA) was not performed and may help to better understand the role of IL-36α and IL-38 in the pathophysiology of T2D and DNP. Finally, other cytokines of the IL-1 family were not analyzed, and simultaneous exploration of these cytokines may contribute to a better understanding of their relationship to low-grade inflammation in T2DM.
In conclusion, the concentrations of IL-36α and IL-38 were up-regulated in the serum of T2D and DNP patients. Both cytokines were proposed to be potential discriminating biomarkers associated with increased risk of T2D and DNP. Targeting the axis of their interaction with other cytokines of the IL-1 family may be important for understanding the pathophysiology of T2D and DNP.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ADA:
-
American Diabetes Association
- ALP:
-
Alkaline phosphatase
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- AUC:
-
Area under the curve
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- DNP:
-
Diabetic neuropathy
- FPG:
-
Fasting plasma glucose
- HbA1c:
-
Glycosylated hemoglobin
- HC:
-
Healthy controls
- HDL:
-
High-density lipoprotein
- HP:
-
High production
- IL:
-
Interleukin
- IQR:
-
Interquartile range
- LDL:
-
Low-density lipoprotein
- LP:
-
Low production
- OR:
-
Odds ratio
- p :
-
Probability
- ROC:
-
Receiver operating characteristic
- rs :
-
Correlation coefficient
- T2D:
-
Type 2 diabetes mellitus without diabetic neuropathy
- T2DM:
-
Type 2 diabetes mellitus
- TC:
-
Total cholesterol
- TCNS:
-
Toronto Clinical Neuropathy Scoring System
- TG:
-
Triglycerides
- TGF:
-
Transforming growth factor
- TNF:
-
Tumor necrosis factor
- WHR:
-
Waist-to-hip ratio
References
Wu H, Patterson CC, Zhang X, Ghani RBA, Magliano DJ, Boyko EJ et al (2022) Worldwide estimates of incidence of type 2 diabetes in children and adolescents in 2021. Diabetes Res Clin Pract 185:109785. https://doi.org/10.1016/J.DIABRES.2022.109785
Feldman EL, Callaghan BC, Pop-Busui R, Zochodne DW, Wright DE, Bennett DL et al (2019) Diabetic neuropathy. Nat Rev Dis Prim 5:41. https://doi.org/10.1038/s41572-019-0092-1
Galicia-Garcia U, Benito-Vicente A, Jebari S, Larrea-Sebal A, Siddiqi H, Uribe KB et al (2020) Pathophysiology of type 2 diabetes mellitus. Int J Mol Sci 21:1–34. https://doi.org/10.3390/ijms21176275
Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Al J, Kaabi (2020) Epidemiology of type 2 diabetes Global Burden of Disease and forecasted trends. J Epidemiol Glob Health. 10:107–11. https://doi.org/10.2991/JEGH.K.191028.001
Berbudi A, Rahmadika N, Tjahjadi AI, Ruslami R (2019) Type 2 diabetes and its impact on the immune system. Curr Diabetes Rev 16:442–9. https://doi.org/10.2174/1573399815666191024085838
Zheng H, Sun W, Zhang Q, Zhang Y, Ji L, Liu X et al (2021) Proinflammatory cytokines predict the incidence of diabetic peripheral neuropathy over 5 years in Chinese type 2 diabetes patients: a prospective cohort study. EClinicalMedicine 31:100649. https://doi.org/10.1016/j.eclinm.2020.100649
Turner MD, Nedjai B, Hurst T, Pennington DJ (2014) Cytokines and chemokines: at the crossroads of cell signalling and inflammatory disease. Biochim Biophys Acta - Mol Cell Res 1843:2563–82. https://doi.org/10.1016/j.bbamcr.2014.05.014
Huang G, Li M, Tian X, Jin Q, Mao Y, Li Y (2022) The emerging roles of IL-36, IL-37, and IL-38 in diabetes mellitus and its complications. Endocrine, Metab Immune Disord - Drug Targets 22:997–1008. https://doi.org/10.2174/1871530322666220113142533
Herder C, Carstensen M, Ouwens DM (2013) Anti-inflammatory cytokines and risk of type 2 diabetes. Diabetes, Obes Metab 15:39–50. https://doi.org/10.1111/dom.12155
Li D, Zhong J, Zhang Q, Zhang J (2023) Effects of anti-inflammatory therapies on glycemic control in type 2 diabetes mellitus. Front Immunol 14:1125116. https://doi.org/10.3389/fimmu.2023.1125116
Velikova TV, Kabakchieva PP, Assyov YS, Georgiev TA (2021) Targeting inflammatory cytokines to improve type 2 diabetes control. Biomed Res Int 2021:7297419. https://doi.org/10.1155/2021/7297419
Frühbeck G, Gómez-Ambrosi J, Ramírez B, Mentxaka A, Rodríguez A, Becerril S et al (2022) Increased levels of interleukin-36 in obesity and type 2 diabetes fuel adipose tissue inflammation by inducing its own expression and release by adipocytes and macrophages. Front Immunol 13:832185. https://doi.org/10.3389/fimmu.2022.832185
Li Y, Chen S, Zhao T, Li M (2021) Serum IL-36 cytokines levels in type 2 diabetes mellitus patients and their association with obesity, insulin resistance, and inflammation. J Clin Lab Anal 35:e23611. https://doi.org/10.1002/jcla.23611
Gurău F, Silvestrini A, Matacchione G, Fazioli F, Bonfigli AR, Olivieri F et al (2021) Plasma levels of interleukin-38 in healthy aging and in type 2 diabetes. Diabetes Res Clin Pract 171:108585. https://doi.org/10.1016/j.diabres.2020.108585
Liu Y, Chen T, Zhou F, Mu D, Liu S (2020) Interleukin-38 increases the insulin sensitivity in children with the type 2 diabetes. Int Immunopharmacol. 82:106264. https://doi.org/10.1016/j.intimp.2020.106264
Chakraborty R, Parveen R, Varshney P, Kapur P, Khatoon S, Saha N et al (2021) Elevated urinary IL-36α and IL-18 levels are associated with diabetic nephropathy in patients with type 2 diabetes mellitus. Minerva Endocrinol 46:226–32. https://doi.org/10.23736/S2724-6507.20.03196-X
Bassoy EY, Towne JE, Gabay C (2018) Regulation and function of interleukin-36 cytokines. Immunol Rev 281:169–78. https://doi.org/10.1111/imr.12610
Haghshenas MR, Zamir MR, Sadeghi M, Fattahi MJ, Mirshekari K, Ghaderi A (2022) Clinical relevance and therapeutic potential of IL-38 in immune and non-immune-related disorders. Eur Cytokine Netw 33:54–69. https://doi.org/10.1684/ecn.2022.0480
Xie L, Huang Z, Li H, Liu X, Zheng S, Su W (2019) IL-38: a new player in inflammatory autoimmune disorders. Biomolecules 9:345. https://doi.org/10.3390/biom9080345
Wang M (2021) The role of IL-37 and IL-38 in obstetrics abnormalities. Front Med 8:737084. https://doi.org/10.3389/fmed.2021.737084
Bril V, Tomioka S, Buchanan RA, Perkins BA (2009) Reliability and validity of the modified Toronto Clinical Neuropathy Score in diabetic sensorimotor polyneuropathy. Diabet Med 26:240–6. https://doi.org/10.1111/j.1464-5491.2009.02667.x
American-Diabetes-Association (2020) Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care 43:S14-31. https://doi.org/10.2337/dc20-S002
Czernichow S, Kengne AP, Stamatakis E, Hamer M, Batty GD (2011) Body mass index, waist circumference and waist-hip ratio: which is the better discriminator of cardiovascular disease mortality risk? Evidence from an individual-participant meta-analysis of 82864 participants from nine cohort studies. Obes Rev 12:680–7. https://doi.org/10.1111/j.1467-789X.2011.00879.x
Kang H (2021) Sample size determination and power analysis using the G*Power software. J Educ Eval Health Prof 18:17. https://doi.org/10.3352/JEEHP.2021.18.17
Aras M, Tchang BG, Pape J (2021) Obesity and diabetes. Nurs Clin North Am 56:527–41. https://doi.org/10.1016/j.cnur.2021.07.008
Klein S, Gastaldelli A, Yki-Järvinen H, Scherer PE (2022) Why does obesity cause diabetes? Cell Metab 34:11–20. https://doi.org/10.1016/j.cmet.2021.12.012
Catalan-Dibene J, McIntyre LL, Zlotnik A (2018) Interleukin 30 to interleukin 40. J Interf Cytokine Res 38:423–39. https://doi.org/10.1089/jir.2018.0089
Boutet MA, Nerviani A, Lliso-Ribera G, Lucchesi D, Prediletto E, Ghirardi GM et al (2020) Interleukin-36 family dysregulation drives joint inflammation and therapy response in psoriatic arthritis. Rheumatol (United Kingdom) 59:828–38. https://doi.org/10.1093/rheumatology/kez358
Leon G, Hussey S, Walsh PT (2021) The diverse roles of the IL-36 family in gastrointestinal inflammation and resolution. Inflamm Bowel Dis 27:440–50. https://doi.org/10.1093/ibd/izaa232
Wang XR, Xiao JP, Wang DG (2021) Elevated levels of serum IL-36α in patients with systemic lupus erythematosus. Biomed Reports 15:76. https://doi.org/10.3892/br.2021.1452
Aravindhan V, Bobhate A, Sathishkumar K, Viswanathan V (2022) Serum levels of novel anti-inflammatory cytokine interleukin-38 in diabetes patients infected with latent tuberculosis (DM-LTB-3). J Diabetes Complications 36:108133. https://doi.org/10.1016/j.jdiacomp.2022.108133
Yu Z, Liu J, Zhang R, Huang X, Sun T, Wu Y et al (2017) IL-37 and 38 signalling in gestational diabetes. J Reprod Immunol 124:8–14. https://doi.org/10.1016/j.jri.2017.09.011
Tsalamandris S, Antonopoulos AS, Oikonomou E, Papamikroulis GA, Vogiatzi G, Papaioannou S et al (2019) The role of inflammation in diabetes: current concepts and future perspectives. Eur Cardiol Rev 14:50–9. https://doi.org/10.15420/ecr.2018.33.1
Vasanthakumar A, Kallies A (2019) Interleukin (Il)-33 and the il-1 family of cytokines—regulators of inflammation and tissue homeostasis. Cold Spring Harb Perspect Biol 11:a028506. https://doi.org/10.1101/cshperspect.a028506
Evavold CL, Kagan JC (2022) Diverse control mechanisms of the interleukin-1 cytokine family. Front Cell Dev Biol 10:910983. https://doi.org/10.3389/fcell.2022.910983
Kelley JB, Talley AM, Spencer A, Gioeli D, Paschal BM (2010) Karyopherin α7 (KPNA7), a divergent member of the importin α family of nuclear import receptors. BMC Cell Biol 11:1–12. https://doi.org/10.1186/1471-2121-11-63
Korwek Z, Tudelska K, Nałȩcz-Jawecki P, Czerkies M, Prus W, Markiewicz J et al (2016) Importins promote high-frequency NF-ΚB oscillations increasing information channel capacity. Biol Direct 11:61. https://doi.org/10.1186/s13062-016-0164-z
Matsumori A (2023) Nuclear factor-κB is a prime candidate for the diagnosis and control of inflammatory cardiovascular disease. Eur Cardiol Rev 18:e40. https://doi.org/10.15420/ecr.2023.10
Suryavanshi SV, Kulkarni YA (2017) NF-κβ: a potential target in the management of vascular complications of diabetes. Front Pharmacol 8:798. https://doi.org/10.3389/fphar.2017.00798
Acknowledgements
The authors appreciate the cooperation of the medical staff and health co-workers at the National Diabetes Center, (Mustansiriyah University) and the National Blood Transfusion Center in Baghdad.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
SWN and INS contributed to laboratory work, data handling, writing, and revising the manuscript. AHA managed data, carried out statistical analyses, and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The participants provided their written informed consent to be included in the study. The study protocol was approved by the Research Ethics Committee of the College of Science, University of Baghdad (Reference No. CSEC/0122/0021 on January 20, 2022).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nassurat, S.W., Salman, I.N. & Ad’hiah, A.H. Association of interleukin-36α and interleukin-38 with type 2 diabetes mellitus and diabetic neuropathy. Egypt J Intern Med 36, 24 (2024). https://doi.org/10.1186/s43162-024-00289-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43162-024-00289-6