Abstract
Background
Evaluation of hypercalcaemia in a patient with chronic kidney disease (CKD) is challenging, especially in low-resource settings. Hormone assays should be interpreted with caution as CKD affects both parathyroid hormone (PTH) and vitamin D. Therapies such as bisphosphonates are contraindicated in CKD, while fluid resuscitation can lead to volume overload. We report the diagnostic workup of a patient with stage V CKD who presented with symptomatic hypercalcaemia and discuss the diagnostic pitfalls and therapeutic challenges.
Case presentation
A 72-year-old Sri Lankan woman with stage V, non-oliguric CKD presented with a 2-week history of worsening lassitude, increased thirst and constipation. She was clinically euvolemic and did not have signs of uraemia. Bilateral lung fields had occasional coarse crepitations. The rest of the physical examination was normal. Her serum creatinine level was similar to her baseline (4.7 mg/dl, eGFR 9 ml/min). She was found to have a high serum calcium (14.3 mg/dl) and phosphate (5.0 mg/dl) levels. Her PTH level was 24.1 pg/ml (15–68), and she was deficient in 25-hydroxycholecalciferol (9 mg/ml (30–100)). She was not on calcium or vitamin D supplementation. Disseminated tuberculosis was diagnosed after detecting granulomata in the lungs and abdomen in the contrast-enhanced computed tomography (CECT) and mycobacterial DNA in sputum. She was hydrated with 0.9% NaCl with meticulous use of frusemide. The effect of frusemide waned off by the 10th day, requiring haemodialysis to control the hypercalcaemia. Vitamin D was replaced intramuscularly with 200,000 IU, after which the calcium levels increased. She was treated with IV pamidronate 30 mg, and the calcium levels started reducing drastically. Antituberculous therapy (ATT) was initiated 7 days after pamidronate treatment. The calcium levels normalised 2 days after ATT and sustained beyond 2 months.
Conclusion
Interpretation of PTH and phosphate levels should be done with caution when evaluating hypercalcaemia in patients with advanced chronic kidney disease. First- and second-generation assays detect PTH fragments which accumulate in CKD, leading to false positives. Hypophosphataemic effects of PTH/PTHrP can be masked by accumulation of phosphate in CKD. Bisphosphonates might have a role in treating calcitriol-induced hypercalcaemia, although this needs further evaluation.
Similar content being viewed by others
Background
Common causes for hypercalcaemia among adults are primary hyperparathyroidism and hypercalcaemia of malignancy. Chronic kidney disease (CKD) makes the diagnostic work-up and treatment of hypercalcaemia challenging. Parathyroid hormone (PTH) assays and vitamin D levels should be interpreted with caution. Therapeutics of hypercalcaemia which includes vigorous intravenous fluid therapy and bisphosphonates can have effects on the chronic kidney disease.
We report a patient with stage 5 CKD who presented with symptomatic hypercalcaemia. We discuss the diagnostic challenges and pitfalls of interpreting the laboratory investigations. Furthermore, we discuss how we managed the patient during the process of evaluation with emphasis on disease-disease and drug-disease interactions. We believe that this case report will provide guidance for clinicians managing hypercalcaemia in patients with advanced CKD.
Case presentation
A 72-year-old female with non-oliguric stage V chronic kidney disease (CKD) secondary to diabetic nephropathy with a baseline serum creatinine of 4.7 mg/dl (eGFR 9 ml/min) presented with a 2-week history of worsening lassitude, malaise, increased thirst and constipation. She did not have fever, chronic cough or a backache; the rest of the systemic inquiry was unremarkable. Her comorbidities included hypertension. Her routine medications were cilnidipine 10 mg daily per oral (PO), tolbutamide 250 mg thrice daily PO, famotidine 20 mg twice daily PO, hydrochlorothiazide 25 mg in the morning PO, prazosin 5 mg thrice daily PO and subcutaneous erythropoietin 4000 IU thrice a week. She was not on calcium supplements, vitamin D or vitamin D analogues.
On examination, the patient was euvolemic. Blood pressure was 160/100 mmHg. Occasional coarse crepitations were heard in both lung fields. There were no flapping tremors, pericardial rubs or conjunctival pallor, palpable lymph nodes or hepatosplenomegaly. The rest of the physical examination was normal.
A blood urea and serum creatinine levels were similar to her baseline values. The evaluation of serum electrolytes revealed a high serum calcium level (Table 1).
Hydrochlorothiazide was withheld, as it can cause hypercalcaemia. Despite stopping the thiazides, the hypercalcaemia persisted. Therefore, she was hydrated with IV 0.9% NaCl (3 l/day). As she had advanced chronic kidney disease, IV frusemide (40 mg twice daily) was used judiciously to prevent volume overload and to increase the urinary calcium excretion. Although the serum calcium levels reduced initially, it increased again requiring treatment with haemodialysis (with a dialysate Ca2+ concentration of 7.21 mg/dl) and IV pamidronate 30 mg single dose (Fig. 1). The calcium levels dropped drastically after the treatment with bisphosphonates.
The investigation results of hypercalcaemia evaluation is given in Table 2.
Vitamin D deficiency was treated with a loading dose of IM vitamin D 200,000 IU and daily vitamin D 5000 IU supplementation.
Activated vitamin D (1,25-dihydroxycholecalciferol) level, serum ACEI levels and PTHrP (parathyroid hormone-related peptide) levels and an uptake scan of the parathyroids were not done due to resource limitation.
A contrast-enhanced computer tomography (CECT) of the neck, chest, abdomen and pelvis was done to identify an occult tumour which revealed widespread granulomata suggestive of tuberculosis. However, granulomata in the bone were not seen. Mycobacterial DNA was detected in the sputum using a cartridge-based nucleic acid amplification test.
A diagnosis of “disseminated miliary tuberculosis leading to hypercalcaemia” was made. She was initiated on anti-tuberculosis therapy (ATT) 7 days after treatment with IV pamidronate. The calcium levels normalised 2 days after starting ATT and persisted beyond 2 months.
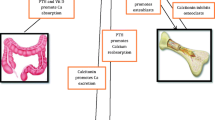
The fluctuation of calcium levels along with the timeline of treatment is given in Fig. 1.
Discussion and conclusion
Evaluation of hypercalcaemia in a patient with chronic kidney disease CKD is challenging, especially in the low-resource setting. We discuss the diagnostic and therapeutic challenges faced in managing a patient with stage V chronic kidney disease, presenting with symptomatic hypercalcaemia.
Chronic kidney disease itself affects the calcium and phosphate metabolism of the body. Hence, the presence of CKD was a major confounding factor in evaluation of the index patient.
What happens to the calcium and phosphate metabolism in chronic kidney disease?
With the progressive loss of nephrons in CKD, the kidney loses its ability to excrete the phosphate ions, which leads to phosphate retention and hyperphosphatemia [1]. Hyperphosphatemia causes increased secretion of fibroblast growth factor 23 (FGF-23) from osteocytes and osteoblasts [2].
FGF-23 have a myriad of effects on the calcium and phosphate metabolism. It reduces phosphate reabsorption in the kidney and increases urinary excretion of phosphate by downregulation of luminal type 2 sodium-dependent phosphate cotransporters in the proximal tubule [3]. In addition, it increases calcium and sodium reabsorption at the distal tubule by increasing expression of apical epithelial calcium channel TRPV5 (transient receptor potential vanilloid-5) and the sodium-chloride cotransporter [4]. Furthermore, FGF-23 reduces synthesis and increases degradation of 1,25-dihydroxycholecalciferol (1,25(OH)2D3) by inhibition of the 1-α-hydroxylase (CYP27B1) and by stimulation of 24-hydroxylase (CYP24A1) respectively [5]. FGF-23 directly reduces production and secretion of PTH acting on PTH transcription [6] and indirectly via increasing calcium-sensing receptor (CaSR) and vitamin D receptor (VDR) expression in the parathyroid glands [6]. It acts on the gut and reduces intestinal absorption of phosphorus through inhibition of NaPi2b cotransporter activity [7].
Hence. the cumulative effect of FGF-23 is to reduce the serum phosphate levels. In early CKD, FGF-23 maintains normophosphataemia by the above mechanisms. However, with the progression of CKD (when GFR declines below 30–40 ml/min/1.73 m2), FGF-23 fails to maintain normophosphataemia, and the serum phosphate levels rise leading to a secondary hyperparathyroidism.
Therefore, advanced chronic kidney disease is a state with normal or low calcium levels, elevated phosphate levels, elevated PTH levels, reduced activated vitamin D3 (1,25(OH)2D3) levels and elevated FGF-23 levels.
As the index patient was not on calcium-based phosphate binders or vitamin D supplementation, we expected the above biochemical/endocrine profile to be unaltered and reflect her baseline state. It posed a difficulty in interpreting the serum phosphate levels when she presented with hypercalcaemia as her baseline serum phosphate level was not known. In an otherwise healthy patient presenting with hypercalcaemia, a low serum phosphate level indicates that the hypercalcaemia is PTH/PTHrP mediated. Such inferences cannot be readily made in the presence of chronic kidney disease. Therefore, we could not narrow down the differential diagnosis with the electrolytes alone.
What causes hypercalcaemia in CKD?
Hypercalcaemia in chronic kidney disease is most commonly due to use of calcium and activated vitamin D supplementation. Prolonged secondary hyperparathyroidism can lead to autonomous secretion of PTH from the parathyroid cells resulting in tertiary hyperparathyroidism.
The index patient was not treated with vitamin D analogues. Her serum PTH levels were not elevated. The imaging of the parathyroids was normal. Hence, both of above possibilities were refuted.
How to interpret the PTH levels of the index patient?
The PTH and vitamin D levels of the patient were tested on the 12th day after admission, due to limitation of resources. Her PTH level was within the normal range, and she was deficient in 25-hydroxycholecalciferol. We expected the PTH levels to be high if it was a PTH-mediated hypercalcaemia and the PTH to be suppressed if it was non-PTH mediated. Interpreting the non-suppressed PTH posed a diagnostic challenge.
The albumin-corrected calcium level and serum phosphate level were 11.9 mg/dl (8.5–10) and 4.3 mg/dl (2.5–4.6), respectively, on the day of the PTH assay (the reduction of phosphate levels from the admission value of 5.0 mg/dl is probably due to the phosphaturic effect of furosemide [8]. The patient was not haemodialysed by this time).
Factors that increase or decrease the secretion of PTH are given in Table 3 [9].
The index patient was hypercalcaemic and normophosphataemic at the time of testing serum PTH levels. Although we do not know the 1,25-dihydroxycholecalciferol levels, it was likely to be elevated as she had a widespread granulomata which secrete activated vitamin D. Therefore, it is expected for her PTH levels to be suppressed (the half-life of PTH is 3 min [10]).
Parathyroid hormone is secreted to the blood as an 84 amino acid peptide, which is biologically active (1–84 PTH). The first 34 amino acids which forms into an alpha helix is crucial for its biological activity [11]. PTH is metabolised in the liver and kidneys into inactive metabolites by the removal of the biologically active N-terminal amino acids [12]. These metabolites are called middle and carboxyl terminal (C terminal) fragments. The abundant fragment is the “7–84 PTH fragment” [13]. These inactive metabolites are excreted via the kidneys, and they tend to accumulate in the body in renal failure. Additionally, parathyroid glands secrete a certain amount of inactive C-terminal PTH to the blood [14].
Parathyroid hormone assays have evolved in three generations. First-generation PTH assays use a single polyclonal antibody directed against the C-terminal part or midterminal part of PTH; hence, it cannot be used to differentiate active PTH from inactive PTH metabolites [15]. Second-generation assays (a sandwich immunoassay) use two antibodies to measure PTH. One antibody is directed against the C-terminal part, and the other antibody is directed against the N-terminal part of PTH. Although the 2nd generation assays are supposed to measure the full length (1–84) PTH, the N-terminal antibody is not directed against the 1st four amino acids. Hence, it can still measure certain PTH fragments, including the 7–84 PTH fragment [13]. Third-generation assays circumvent this problem by using antibodies, directed against the first four amino acids of full-length PTH. Although third-generation assays seem more specific than second-generation assays, these assays can detect PTH forms that are posttranslationally modified, like the form found overproduced in parathyroid carcinoma [15].
We used a second-generation PTH assay. Hence, it might be that the PTH was actually suppressed, but the assay measured PTH fragments, which were accumulated due to the chronic kidney disease. Unavailability of a third-generation assay was a major limitation of the evaluation. A parathyroid uptake scan can be used to detect the activity of the glands. Absent or reduced uptake by the parathyroids would confirm the conclusion that the unexpectedly normal level of PTH of the index patient was due to assay interference by metabolites of PTH.
Therefore, when interpreting the PTH levels in patients with advanced CKD, it is prudent to be mindful about the generation of the assay. Furthermore, higher generation assays should be used upfront, and there should be a low threshold for parathyroid uptake scans.
The patient was then evaluated for a non-PTH-mediated cause for hypercalcaemia, which led to a diagnosis of disseminated tuberculosis.
How does tuberculosis cause hypercalcaemia?
Hypercalcaemia is known to occur in granulomatous diseases (tuberculosis, sarcoidosis, leprosy, lymphomas, berylliosis and disseminated candidiasis), and it is mediated by increased production of activated vitamin D3 (1,25-dihydroxycholecalciferol) [16]. As hypercalcaemia occurs in anephric patients with granulomatous diseases, production of 1,25-dihydroxycholecalciferol should be extra-renal [17, 18]. Pulmonary alveolar macrophages in sarcoidosis [19] and tuberculosis [20] express 25(OH)D3-1α-hydroxylase. Therefore, hypercalcaemia in granulomatous diseases is mediated by enhanced production of 1,25-dihydroxycholecalciferol by activated macrophages and T lymphocytes within the granulomata. It is believed that vitamin D promotes intracellular killing of mycobacteria by macrophages [20, 21].
Unlike the renal 1α-hydroxylase, the counterpart enzyme expressed in granulomata is not inhibited by calcium or 1,25-dihydroxycholecalciferol [19] and is stimulated by interferon ϒ, but not PTH [22]. Hence, production of 1,25-dihydroxycholecalciferol is proportional to the availability of 25-hydroxycholecalciferol in granulomatous diseases.
It can be seen that the serum calcium levels increased after replacement of vitamin D in the index patient, despite treatment with furosemide.
Why was the patient treated with vitamin D? Was it right?
Replacement of vitamin D should be considered in cases of primary hyperparathyroidism-mediated hypercalcaemia for a multitude of reasons. Primary hyperparathyroidism is associated with vitamin D (25-hydroxycholecalciferol) deficiency [23, 24], and the combination indicates a poor outcome. Patients with vitamin D deficiency and primary hyperparathyroidism have higher PTH levels, higher bone turnover, reduced bone mineral density (BMD) and higher incidence of postoperative hypocalcaemia, compared to patients with primary hyperparathyroidism without vitamin D deficiency [25,26,27]. A randomised controlled clinical trial with 46 patients revealed that perioperative vitamin D supplementation in patients with primary hyperparathyroidism and vitamin D deficiency lead to reduced PTH levels and reduced bone resorption with improved bone mineral density before surgery [28]. Interestingly, the vitamin D deficiency, associated with hyperparathyroidism, resolves after parathyroid surgery even without vitamin supplementation [29].
In the index patient, vitamin D deficiency was noted before the detection of disseminated tuberculosis, while a PTH-mediated hypercalcaemia was still being considered. In retrospect, replacement of vitamin D turned out to be detrimental. Therefore, prompt vitamin D replacement should be discouraged in hypercalcaemic patients, who do not have clear evidence of hyperparathyroidism.
Treatment with fluids and frusemide — was it right? And why did it fail?
Loop diuretics increase urinary calcium excretion and can be used in the treatment for acute hypercalcaemia [30]. The 2022 Endocrine Society clinical practice guidelines for treatment of hypercalcaemia of malignancy state that frusemide can be added to fluid hydration in hypercalcaemia, although “there is very little evidence to support the efficacy and safety of the use in the management of hypercalcaemia of malignancy” [31]. Loop diuretics should be used with caution as hypercalcaemia itself cause a nephrogenic diabetes insipidus. Use of frusemide without appropriate fluid replacement can lead to dehydration and worsen hypercalcaemia. The patient in this case had advanced chronic kidney disease; hence, she was at risk of getting overloaded with fluid resuscitation. Therefore, we used frusemide to prevent hypervolaemia and used its hypercalciuric effects to mitigate the hypercalcaemia.
In the index case as there was a diagnostic delay, the patient was treated with a prolonged course of loop diuretics where it was noted that the effect of frusemide diminished beyond the 10th day. The patient was not clinically volume deplete at any point, and the total fluid intake was matched to the previous day’s urine output plus the insensible losses. Hence, the persistence of hypercalcaemia despite frusemide is unlikely to be due to urinary free water loss resulting in increased concentration of the serum calcium. The serum creatinine did not change drastically throughout this period. We do not know the underlying mechanism for the reducing efficacy of frusemide with time. It is possible that the kidneys increased their capacity to reabsorb Ca2+ from the distal tubules over time, as demonstrated in rat models. In a study where mice were treated with frusemide, it was noted that chronic furosemide administration was associated with elevated mRNA levels for calcium transport molecules (TRPV5, TRPV6) and storage molecules (calbindin-D28k and calbindin-D9k) in the renal tubular cells [32].
Bisphosphonates for 1,25-dihydroxycholecalciferol-mediated hypercalcaemia
As the patient’s response to frusemide was declining over time and the calcium levels were increasing progressively requiring haemodialysis, she was treated with IV pamidronate while evaluating for a possible aetiology, in consideration of possible PTH-mediated disease. We had to resort to bisphosphonates accepting the renal toxicity despite the patient having CKD, because denosumab was not available. It was noted that the calcium levels started dropping after the treatment with bisphosphonates.
Vitamin D causes hypercalcaemia by predominantly increasing the gut absorption and renal reabsorption (via TRPV-5 in the proximal tubule) of calcium [33]. The effect of vitamin D3 on bone is mainly to activate osteoblasts directly and osteoclasts indirectly, leading to increased bone matrix deposition.
Bisphosphonates cause osteoclast apoptosis and reduce bone resorption [34]. Therefore, bisphosphonates are advocated in hypercalcaemia secondary to increased bone resorption, typically in hypercalcaemia secondary to malignancy. Are bisphosphonates effective in treating activated vitamin D3-mediated hypercalcaemia?
There are no randomised clinical trials that have evaluated the therapeutic efficacy of bisphosphonates in vitamin D3-mediated hypercalcaemia. However, there are case reports which have demonstrated a reduction in calcium levels after treatment with bisphosphonates in granulomatous diseases.
Issacs et al. treated a patient with miliary tuberculosis with 3-amino-l-hydroxypropylidene-1,I-biphosphonate 0.3 mg/kg on two consecutive days, prior to starting anti-tuberculosis treatment which resulted in a “prompt fall in serum calcium levels” [35]. Roselyn et al. treated a patient with vitamin D3-induced hypercalcaemia secondary to a follicular lymphoma with IV zolendronic acid and successfully maintained normo-calcaemia until initiation of chemotherapy [36]. The 2022 “endocrine society clinical practice guidelines for treatment of hypercalcemia of malignancy in adults” recommend bisphosphonates for calcitriol-mediated hypercalcaemia (as in lymphomas), if the patients continue to have severe or symptomatic hypercalcemia despite treatment with glucocorticoids [31].
In the index patient, it was noted that the serum calcium levels started to normalise with the treatment with bisphosphonates. She was not treated with steroids at any point. The CECT did not reveal any tuberculous granulomata in the bone, which would have otherwise explained the response to bisphosphonates. A bone scan was not done due to the limited resources. Therefore, bisphosphonates might be effective in treating hypercalcaemia in granulomatous diseases. Its efficacy and the probable underlying mechanism should be studied further.
The use of bisphosphonates in advanced CKD is generally contraindicated due to inadequate data and concerns of risk. The risks of bisphosphonates in advanced chronic kidney disease are twofold: renal toxicity and the adverse effects on bone. Bisphosphonates can cause focal segmental glomerulosclerosis [37] and acute tubular necrosis [38]. In the bone, it can cause low bone turnover, osteomalacia mixed uraemic osteodystrophy and worsening of hyperparathyroidism when used in patients with CKD [39].
However, the patient’s kidney function remained stable 1 week after treatment with bisphosphonates. The long-term effects of using bisphosphonates in this patient are yet to be unfolded.
In conclusion, evaluating hypercalcaemia in patients with CKD is challenging. The serum phosphate levels should be interpreted with caution, as they are elevated due to CKD per se. Reduced renal functions can lead to accumulation of PTH fragments, which can give a falsely elevated PTH levels, especially if older generation assays are used. Third-generation assays and parathyroid uptake scans are preferred. The hypocalcaemic effect of furosemide may be less in advanced kidney disease and could fade with continuous treatment. Although granulomatous diseases cause a vitamin D-mediated hypercalcaemia, bisphosphonates seem to be effective in reducing calcium levels; further studies are needed to elucidate the efficacy and probable mechanism.
The serum calcium levels dropped with the initial treatment with fluid resuscitation and use of IV frusemide, although this effect waned off by the 10th day. Haemodialysis was used to control the worsening hypercalcemia. Note how the calcium levels increased after replacing vitamin D, despite haemodialysis. Calcium levels started reducing after treatment with bisphosphonates and plummeted to the normal range after initiating antituberculous therapy.
Availability of data and materials
Not applicable.
Abbreviations
- 1,25(OH)2D3:
-
1,25-Dihydroxycholecalciferol
- ALP:
-
Alkaline phosphatase
- ATT:
-
Antituberculous therapy
- CaSR:
-
Calcium-sensing receptor
- CECT:
-
Contrast-enhanced computer tomography
- CKD:
-
Chronic kidney disease
- ESR:
-
Erythrocyte sedimentation rate
- FGF-23:
-
Fibroblast growth factor 23
- PO:
-
Per oral
- PTH:
-
Parathyroid hormone
- PTHrP:
-
Parathyroid hormone-related peptide
- TRPV:
-
Transient receptor potential vanilloid
- VDR:
-
Vitamin D receptor
References
Hu L, Napoletano A, Provenzano M, Garofalo C, Bini C, Comai G et al (2022) Mineral bone disorders in kidney disease patients: the ever-current topic. Int J Mol Sci. 23:12223
Isakova T, Wahl P, Vargas GS, Gutiérrez OM, Scialla J, Xie H et al (2011) Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int 79:1370–1378
Hu MC, Shi M, Moe OW (2018) Role of αKlotho and FGF23 in regulation of type II Na-dependent phosphate co-transporters. Pflügers Arch - Eur J Physiol. 471:99–108
Andrukhova O, Smorodchenko A, Egerbacher M, Streicher C, Zeitz U, Goetz R et al (2014) FGF23 promotes renal calcium reabsorption through the TRPV5 channel. EMBO J 33:229–246
Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y et al (2004) FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res 19:429–435
Canalejo R, Canalejo A, Martinez-Moreno JM, Rodriguez-Ortiz ME, Estepa JC, Mendoza FJ et al (2010) FGF23 fails to inhibit uremic parathyroid glands. J Am Soc Nephrol 21:1125–1135
Miyamoto KI, Ito M, Kuwahata M, Kato S, Segawa H (2005) Inhibition of intestinal sodium-dependent inorganic phosphate transport by fibroblast growth factor 23. Ther Apher Dial 9:331–335
Liamis G, Milionis HJ, Elisaf M (2010) Medication-induced hypophosphatemia: a review. QJM An Int J Med 103:449–459
Pocotte SL, Ehrenstein G, Fitzpatrick LA (1991) Regulation of parathyroid hormone secretion. Endocr Rev 12:291–301
Leiker AJ, Yen TWF, Eastwood DC, Doffek KM, Szabo A, Evans DB et al (2013) Factors that influence parathyroid hormone half-life: are new intraoperative criteria needed? JAMA Surg 148:602
Marx UC, Adermann K, Bayer P, Meyer M, Forssmann WG, Rösch P (1998) Structure-activity relation of NH2-terminal human parathyroid hormone fragments. J Biol Chem 273:4308–4316
Cavalier E, Delanaye P, Nyssen L, Souberbielle JC (2015) Problems with the PTH assays. Ann Endocrinol (Paris) 76:128–133
Lepage R, Roy L, Brossard JH, Rousseau L, Dorais C, Lazure C et al (1998) A non-(1–84) circulating parathyroid hormone (PTH) fragment interferes significantly with intact PTH commercial assay measurements in uremic samples. Clin Chem 44:805–809
Murray TM, Rao LG, Divieti P, Bringhurst FR (2005) Parathyroid hormone secretion and action: evidence for discrete receptors for the carboxyl-terminal region and related biological actions of carboxyl- terminal ligands. Endocr Rev 26:78–113
Smit MA, Van Kinschot CMJ, Van Der Linden J, Van Noord C, Kos S (2019) Clinical guidelines and PTH measurement: does assay generation matter? Endocr Rev 40:1468–1480
Negri AL, Diez GR, Del Valle E, Piulats E, Greloni G, Quevedo A et al (2014) Hypercalcemia secondary to granulomatous disease caused by the injection of methacrylate: a case series. Clin Cases Miner Bone Metab 11:44
JK M, B V, P NC, S S (1982) Elevated 1,25-dihydroxyvitamin D levels: occurrence with sarcoidosis with end-stage renal disease. Arch Intern Med 142:1206–1207
Barbour GL, Coburn JW, Slatopolsky E, Norman AW, Horst RL (1981) Hypercalcemia in an anephric patient with sarcoidosis: evidence for extrarenal generation of 1,25-dihydroxyvitamin D. N Engl J Med 305:440–443
Adams JS, Gacad MA (1985) Characterization of 1 alpha-hydroxylation of vitamin D3 sterols by cultured alveolar macrophages from patients with sarcoidosis. J Exp Med 161:755–765
Cadranel J, Garabedian M, Milleron B, Guillozo H, Akoun G, Hance AJ (1990) 1,25(OH)2D2 production by T lymphocytes and alveolar macrophages recovered by lavage from normocalcemic patients with tuberculosis. J Clin Invest 85:1588–1593
Scanga CA, Mohan VP, Tanaka K, Alland D, Flynn JL, Chan J (2001) The inducible nitric oxide synthase locus confers protection against aerogenic challenge of both clinical and laboratory strains of Mycobacterium tuberculosis in mice. Infect Immun 69:7711–7717
Tebben PJ, Singh RJ, Kumar R (2016) Vitamin D-mediated hypercalcemia: mechanisms, diagnosis, and treatment. Endocr Rev 37:521–547
Rao DS, Agarwal G, Talpos GB, Phillips ER, Bandeira F, Mishra SK et al (2002) Role of vitamin D and calcium nutrition in disease expression and parathyroid tumor growth in primary hyperparathyroidism: a global perspective. J bone Miner Res Off J Am Soc Bone Miner Res 17(Suppl 2):N75-80
Moosgaard B, Vestergaard P, Heickendorff L, Melsen F, Christiansen P, Mosekilde L (2005) Vitamin D status, seasonal variations, parathyroid adenoma weight and bone mineral density in primary hyperparathyroidism. Clin Endocrinol (Oxf) 63:506–513
Priya G, Jyotsna VP, Gupta N, Chumber S, Bal CS, Karak AK et al (2008) Clinical and laboratory profile of primary hyperparathyroidism in India. Postgrad Med J 84:34–39
Özbey N, Erbil Y, Ademoǧlu E, Özarmaǧan S, Barbaros U, Bozbora A (2006) Correlations between vitamin D status and biochemical/clinical and pathological parameters in primary hyperparathyroidism. World J Surg 30:321–326
Untch BR, Barfield ME, Dar M, Dixit D, Leight GS, Olson JA (2007) Impact of 25-hydroxyvitamin D deficiency on perioperative parathyroid hormone kinetics and results in patients with primary hyperparathyroidism. Surgery 142:1022–1026
Rolighed L, Rejnmark L, Sikjaer T, Heickendorff L, Vestergaard P, Mosekilde L et al (2014) Vitamin D treatment in primary hyperparathyroidism: a randomized placebo controlled trial. J Clin Endocrinol Metab 99:1072–1080
Amstrup AK, Rejnmark L, Mosekilde L (2011) Patients with surgically cured primary hyperparathyroidism have a reduced quality of life compared with population-based healthy sex-, age-, and season-matched controls. Eur J Endocrinol 165:753–760
Suki WN, Yium JJ, Von Minden M, Saller-Hebert C, Eknoyan G, Martinez-Maldonado M (2010) Acute treatment of hypercalcemia with furosemide. N Engl J Med. 283:836–840. https://doi.org/10.1056/NEJM197010152831603
El-Hajj Fuleihan G, Clines GA, Hu MI, Marcocci C, Murad MH, Piggott T et al (2023) Treatment of hypercalcemia of malignancy in adults: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 108:507–528
Te LC, Chen HC, Lai LW, Yong KC, Lien YHH (2007) Effects of furosemide on renal calcium handling. Am J Physiol Renal Physiol. 293:F1231
Lips P, Van Schoor NM (2011) The effect of vitamin D on bone and osteoporosis. Best Pract Res Clin Endocrinol Metab 25:585–591
Drake MT, Clarke BL, Khosla S (2008) Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc 83:1032
Isaacs RD, Nicholson GI, Holdaway IM Miliary tuberculosis with hypercalcaemia and raised vitamin D concentrations. https://doi.org/10.1136/thx.42.7.555
Mateo RCI, Ortiz R, Rosen HN (2019) Bisphosphonates for the treatment of calcitriol-induced hypercalcemia. AACE Clin Case Reports 5:e316
Markowitz GS, Appel GB, Fine PL, Fenves AZ, Loon NR, Jagannath S et al (2001) Collapsing focal segmental glomerulosclerosis following treatment with high-dose pamidronate. J Am Soc Nephrol 12:1164–1172
Banerjee D, Asif A, Striker L, Preston RA, Bourgoignie JJ, Roth D (2003) Short-term, high-dose pamidronate-induced acute tubular necrosis: the postulated mechanisms of bisphosphonate nephrotoxicity. Am J kidney Dis Off J Natl Kidney Found 41:E18
Toussaint N, Elder GJ, Kerr PG (2009) Bisphosphonates in chronic kidney disease; balancing potential benefits and adverse effects on bone and soft tissue. Clin J Am Soc Nephrol 4:221–233
Acknowledgements
None.
Funding
No funding involved.
Author information
Authors and Affiliations
Contributions
All authors were involved in diagnosing and managing the patient. PR drafted the manuscript, while HD and DW critically revised it. DW, EW and RL provided the expertise in nephrology, while HD and PK provided the expertise in endocrinology. SM collected the data and maintained clinical records. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Informed written consent was obtained from the index patient for publishing the clinical details and photographs after anonymising. Approval from an ethics review committee was not sought as the publication is a case report.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the editor-in-chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ruwanpathirana, P., Dissanayaka, H., Munasinghe, S. et al. Diagnostic pitfalls and therapeutic challenges of hypercalcaemia in chronic kidney disease: a case report and a narrative review. Egypt J Intern Med 36, 14 (2024). https://doi.org/10.1186/s43162-024-00278-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43162-024-00278-9