Abstract
Background
Chronic kidney disease (CKD) is a progressive failure of renal function with ongoing systemic inflammation. Inflammatory markers such as neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR), and proteinuria were documented as independent predictors of CKD progression. Although proteinuria estimated by the protein to creatinine ratio (UPCR) is generally employed to screen the disease progression of CKD, the correlation of NLR and PLR with different stages of CKD is yet to be studied. Consequently, this study strived to find the stage-wise correlation between NLR and PLR with proteinuria in CKD patients.
Methods
Eighty-five CKD patients with proteinuria who visited the Nephrology Clinic at Teaching Hospital Jaffna, Sri Lanka, were randomly selected and categorized as stages II to IV based on the estimated glomerular filtration rate (e-GFR). Blood samples were collected and subjected to investigate patients’ NLR and PLR. Furthermore, urine protein and creatinine were measured and UPCR was calculated. Participants’ demographic, clinical, and laboratory data were obtained from patients’ clinical registry. Spearman’s rank correlation and receiver operative characteristic (ROC) curve analysis was done, and the p value < 0.05 was considered statistically significant.
Results
Amongst the total participants, males were predominant (58.8%), with a mean age of 58.1. Severity analysis based on the e-GFR revealed that 17.64%, 18.82%, 29.41%, and 34.11% of CKD patients were in stages II, IIIA, IIIB, and IV, respectively. Stage-wise correlation and ROC curve analysis indicated that NLR and PLR were positively correlated with UPCR in stages IIIA, IIIB, and IV of CKD with more than 80% predictive sensitivity and specificity.
Conclusion
NLR and PLR can be used as novel predictive markers for monitoring the severity of CKD; however, further large-scale cohort studies of NLR and PLR with serial monitoring and multiple closely spaced measurements are recommended to develop these markers into clinically acceptable markers for CKD progression.
Similar content being viewed by others
Background
Chronic kidney disease (CKD), defined by e-GFR below 60 ml/min/1.73 m2 sustained for at least 3 months or higher e-GFR with the existence of proteinuria, affects at least 1 in 10 individuals in the world [1, 2]. Patients with CKD are vulnerable to severe cardiovascular complications, bone diseases, infections, anaemia, and cancer, leading to low life expectancy [3]. In 2019, CKD was documented as the 11th leading cause of death worldwide, accounting for over 1.4 million deaths, and it was further predicted that the death rate would be raised by 2.79-fold in 2040 [4, 5]. There were several risk factors, including hypertension, dyslipidemia, diabetes mellitus, and aging, reported to be associated with CKD [6].
Early detection and monitoring of disease progression is crucial in the treatment of CKD. Proteinuria is one of the common markers used for the severity monitoring of CKD, as it shows a strong association with its progression [7]. In clinical practice, the 24-h urine protein excretion, UPCR, and urinary albumin-creatinine ratio (UACR) are commonly used to assess proteinuria; however, the 24-h urine collection is often inconvenient to the patients and can be inaccurate since the constituent of urine is easily altered by environmental factors [8]. Furthermore, UPCR was encountered to be influenced by muscle mass, urine tonicity, sex, age, dietary conditions, and other coexisting medical conditions [9]. Notably, recent studies supported and recommended the use of spot urine for UPCR calculation instead of a 24-h urine sample [10].
Inflammatory markers were recognised as suitable indicators for monitoring the disease progression of CKD, as inflammatory responses have a vital part in the pathogenesis of end-stage kidney disease [11]. Recently, it was found that inflammatory markers, NLR and PLR, predict the disease progression and the presence of proteinuria in CKD patients [12, 13]. NLR and PLR are the most economical and readily available markers and are easily obtainable from simple mathematical calculations from routine complete blood count tests [14]. Although the NLR and PLR were found to have an association with the pathogenesis of CKD and were suggested as alternative markers for CKD progress monitoring, the correlation between NLR and PLR with proteinuria in distinct phases of the CKD is yet to be revealed. Therefore, the present study attempted to discover the correlation between NLR and PLR with proteinuria in different stages of CKD.
Methods
Patient recruitment and study design
It was a single-center, laboratory-based cross-sectional study. Eighty-five CKD patients with proteinuria (from stages II to IV) who attended the Nephrology Clinic at the Teaching Hospital Jaffna, Sri Lanka, between the 1st of July 2022 and the 31st of September 2022 were recruited for the study. Individuals who appeared with a history of kidney transplantation, coronary diseases and heart failure, recent hospitalization, surgeries, and exposure to non-steroidal anti-inflammatory (NSAID), nephrotoxic and immunosuppressive, steroidal, and antipsychotic drugs within the last one month and patients diagnosed with infection and febrile illness within the last two weeks were excluded from the study.
Data collection
Participants’ basic characteristics of age, gender, associated comorbidities, laboratory test results, and drug prescriptions were extracted from participants’ medical records maintained at the hospital registry, after getting ethical clearance from the Ethics Review Committee of the Faculty of Medicine, University of Jaffna, Sri Lanka, in June 2022. e-GFR was computed by CKD Epidemiology Collaboration Creatinine Equation [(e-GFR = 141 × min(Scr/k,1)α × max(Scr/k,1)−1.209 × 0.993Age × 1.018 (if female) × 1.159 (if black)] [15]. The patients were categorised into stages II to IV according to the National Kidney Foundation guidelines [16].
Blood cell counting and calculation of NLR and PLR
EDTA blood samples were withdrawn from each participant after obtaining informed written consent. Laboratory investigations of white blood cell (WBC), platelet, and leukocyte differential counts were performed by manual technique. Total WBC and platelet count were performed by a method described by Bain et al. [17]. Briefly, 20 µL of blood was mixed with 380 µL of 1% acetic acid and 380 µL of ammonium oxalate, respectively, and subjected to cell counting by a haemocytometer. Leukocyte differential count was done by a protocol published by Bain et al. [17]. Initially, blood smears were prepared and stained by Leishman’s stain, and then, cells were counted under oil immersion in a light microscope. All the cell counting was performed by three laboratory experts individually as five replicates, and the result mean was utilized to calculate NLR and PLR.
Urine protein and creatinine measurement and UPCR calculation
Random (spot) urine samples were collected in a sterile urine collection container and transported to the Chemical Pathology Laboratory at Teaching Hospital Jaffna. Urinary total protein was measured by a Thermo Scientific Konelab 20 clinical chemistry analyzer, whereas urinary creatinine was measured by a Siemens Dimension RxL Max automated analyzer according to manufacturers’ instructions. UPCR was obtained by dividing urinary total protein concentration by urinary creatinine concentration.
Statistical analysis
Statistical analyses were performed by Statistical Package for the Social Science (SPSS) version 18. Numerical data were subjected to check for normal distribution by the Kolmogorov–Smirnov test. The variables that showed normal distribution were presented as mean ± SD and compared by one-way ANOVA, while variables that exhibited non-normal distribution were summarised as medians with interquartile ranges (IQRs) and analyzed using the Kruskal–Wallis H test. All nominal values were illustrated as frequencies and compared by Fisher’s exact test. Correlations were determined by Spearman’s rank correlation. The receiver operating characteristic (ROC) curve and Youden’s index were employed to assess the optimum cutoff for NLR and PLR. p < 0.05 was considered statistically significant.
Results
Baseline characteristics of participants
During the study period, 85 CKD patients with proteinuria who visited the Nephrology Clinic were recruited for the investigation. The mean age of the participants was 58.1, with a male predominance of 58.8%. Most of the patients had multiple comorbidities (28.2%), although hypertension (24.7%), glomerulonephritis (18.8%), and diabetes (10.6%) were the leading comorbidities associated with CKD in the participants (Table 1). Furthermore, 17.65% and 50.59% of the participants were under the medications of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers, respectively. The laboratory data extracted from the patient’s medical record and inflammatory markers are illustrated in Table 1.
Laboratory findings in different stages of CKD
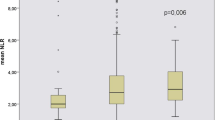
CKD patients were divided into stages II to IV based on the e-GFR. Amongst the total participants, the majority of patients were at stage IV (n = 29), while others were in stages II, IIIA, and IIIB. The laboratory data indicated that serum creatinine (p < 0.0001), UPCR (p < 0.0001), neutrophil count (p < 0.0001), lymphocyte count (p = 0.001), platelet count (p = 0.013), NLR (p < 0.0001), and PLR (p < 0.0001) had significant difference between stages II-IV of CKD (Table 2). Particularly, inflammatory markers such as UPCR, NLR, and PLR were increased from stage II to stage IV.
Correlation between NLR and PLR with Proteinuria in different stages of CKD
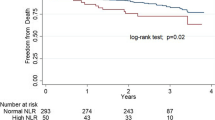
In order to understand the correlation between NLR and PLR with proteinuria, a bivariate correlation analysis was performed. Results revealed that NLR and PLR had statistically significant positive correlations with UPCR and serum creatinine while showing statistically significant negative correlation with eGFR (Table 3). Stage-wise correlation analyses indicated that NLR and PLR had a significant positive correlation with UPCR only in the stages of IIIA, IIIB, and IV of CKD and did not show a significant correlation in stage II (Table 4). Figures 1 and 2 demonstrate the correlation of NLR and PLR with UPCR in stages IIIA, IIIB, and IV of CKD.
Predictive performance of NLR and PLR in CKD progression
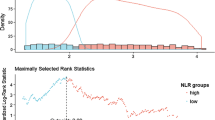
Since NLR and PLR exhibited a positive correlation with UPCR in stages IIIA, IIIB, and IV of CKD, ROC curve analysis and Youden’s index calculation were performed to understand the predictive ability of NLR and PLR. As shown in Fig. 3, the ROC curve illustrates a significantly larger area of NLR and PLR for stages IIIA, IIIB, and IV of CKD with more than 80% sensitivity and specificity for both markers. The optimal cutoff obtained from Youden’s index, area under the curve (AUC), and the predictive accuracy of NLR and PLR are illustrated in Table 5.
Discussion
The present study shows the correlation of NLR and PLR with proteinuria in distinct stages of CKD patients. A statistically significant positive correlation was observed in stages IIIA, IIIB, and IV of CKD between NLR and PLR with proteinuria.
It is widely reported that inflammation has a key role in the progression of kidney disease through oxidative and carbonyl stress [18]. The inflammatory stress of the human body is impacted by antioxidants in the diet, repeated infections, intestinal dysbiosis, modified adipose tissue metabolism, and nutritional imbalances [19]. Furthermore, recent studies revealed that measurement of inflammatory markers could assist in finding the status of CKD as they drive its progression by stimulating unwanted modifications in the function of the glomerulus, including alteration in the selective nature of the glomerular filtration, increasing the extracellular matrix in the glomerular interstitial space, and destruction of the tubular epithelium [20]. Although interleukins 6, 8, 12, and 33, as well as tumour necrotic factor-alpha (TNF-α), erythrocyte sedimentation rate, and C-reactive protein (CRP), were discovered as inflammatory markers, it is still unclear which of the inflammatory makers is the most promising to monitor CKD progression [19, 21, 22]. Previous studies demonstrated that increased neutrophil count had a significant association with oxidative stress, and decreased neutrophil count indicated the deterioration of nutritional status [23, 24]. It was further revealed that oxidative stress and nutritional imbalances had a significant association with CKD progression and abnormal renal outcome, respectively [25, 26]. The findings of the existing study also displayed significant elevations in neutrophil count and reduced lymphocyte count between distinct stages of CKD. Therefore, the results of the existing study are compatible with the previous reports. Similarly, multiple studies indicated that NLR and PLR could be useful for predicting disease progression in CKD patients [27,28,29].
NLR and PLR are efficiently computed from the routine laboratory investigation of complete blood count and are cheaper and easily obtainable compared to other inflammatory markers [30]. A study done by Kocyigit et al. in 2013 among 105 stage IV CKD patients demonstrated that increased levels of NLR were associated with higher baseline CRP and faster decline in GFR leading to kidney failure compared to the individuals with low NLR value [29]. Turkmen et al. showed that PLR could be assumed as a more useful predictive marker for detecting kidney disease as it was elevated and showed a positive correlation with acute phase reactants such as NLR, CRP, interlukin-6, and TNF-α in end-stage CKD patients [31]. Results of the present study showed NLR and PLR were elevated from stages II to IV, which was compatible with the previous studies that demonstrated PLR and NLR were increased with the disease progression in CKD [32,33,34].
Proteinuria is a well-known marker for monitoring CKD severity as it increases with the severity of kidney disease through various pathways, including activation of complement and tubular chemokine expression [35]. Although proteinuria is generally assessed by measuring the UPCR in clinical laboratories, inconvenience caused to the patients during sample collection and its instability due to urine tonicity, sex, age, nutritional conditions, and other associated diseases, it is still challenging to use proteinuria as an independent marker for the monitoring of CKD progression [9]. As a result, the current study attempted to find the correlation between NLR and PLR with proteinuria in stages II–IV of CKD to understand the nature of NLR and PLR in severity monitoring of CKD and the results indicated that NLR and PLR were positively correlated with UPCR in stages IIIA, IIIB, and IV of CKD with more than 80% sensitivity and specificity.
There were several limitations attributed to this study. First, it is a laboratory-based cross-sectional study performed from samples collected from a single hospital treating only the ethnic group living in the Northern part of Sri Lanka; therefore, the generalization of these findings to different ethnicities is still unknown. Second, the sample size of the present study is comparatively small for performing some statistical tests; thus, a larger cohort study needs to be done in the future to find the correlations of NLR and PLR with proteinuria in CKD patients. At last, it may not be accurate and precise to use NLR and PLR for predicting CKD progression using a single measurement of NLR and/or PLR.
Conclusions
The results indicate that NLR and PLR are positively correlated with proteinuria in different stages of CKD. In light of these positive findings, we suggest that the significance of serial monitoring of these markers and the use of multiple (2 to 3), closely spaced measurements of NLR and PLR, should be further studied with a cohort study design, to develop these low-cost markers further into clinically acceptable markers for CKD progression.
Availability of data and materials
All the datasets analyzed in this study are available on request from the corresponding author.
Abbreviations
- CKD:
-
Chronic kidney disease
- NLR:
-
Neutrophil to lymphocyte ratio
- PLR:
-
Platelet to lymphocyte ratio
- e-GFR:
-
Estimated glomerular filtration rate
- UPCR:
-
Urine protein to creatinine ratio
- UACR:
-
Urine albumin to creatinine ratio
- TNF-α:
-
Tumor necrotic factor-alpha
- CRP:
-
C-reactive protein
References
Liyanage T, Toyama T, Hockham C et al (2022) Prevalence of chronic kidney disease in Asia: a systematic review and analysis. BMJ Glob Health 7(1):e007525
Kovesdy CP (2011) (2022) Epidemiology of chronic kidney disease: an update 2022. Kidney Int Suppl 12(1):7–11
Webster AC, Nagler EV, Morton RL, Masson P (2017) Chronic kidney disease. Lancet 389(10075):1238–1252
Foreman KJ, Marquez N, Dolgert A et al (2018) Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016–40 for 195 countries and territories. Lancet 392(10159):2052–2090
Diseases GBD, Injuries C (2020) Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396(10258):1204–1222
Ghelichi-Ghojogh M, Fararouei M, Seif M, Pakfetrat M (2022) Chronic kidney disease and its health-related factors: a case-control study. BMC Nephrol 23(1):24
Tourojman M, Kirmiz S, Boelkins B et al (2016) Impact of reduced glomerular filtration rate and proteinuria on overall survival of patients with renal cancer. J Urol 195(3):588–593
Qin X, Hu H, Cen J, Wang X, Wan Q, Wei Z (2022) Association between urinary protein-to-creatinine ratio and chronic kidney disease progression: a secondary analysis of a prospective cohort study. Front Med 9:854300
Oh YJ, Ro H, Chung W et al (2022) Urine creatinine concentration influences the prognostic value of proteinuria for MACE prediction from the findings of the KNOW-CKD study. Sci Rep 12(1):15924
Kaminska J, Dymicka-Piekarska V, Tomaszewska J, Matowicka-Karna J, Koper-Lenkiewicz OM (2020) Diagnostic utility of protein to creatinine ratio (P/C ratio) in spot urine sample within routine clinical practice. Crit Rev Clin Lab Sci 57(5):345–364
Li P, Xia C, Liu P, Peng Z, Huang H, Wu J, He Z (2020) Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in evaluation of inflammation in non-dialysis patients with end-stage renal disease (ESRD). BMC Nephrol 21(1):511
Yuan Q, Wang J, Peng Z et al (2019) Neutrophil-to-lymphocyte ratio and incident end-stage renal disease in Chinese patients with chronic kidney disease: results from the Chinese Cohort Study of Chronic Kidney Disease (C-STRIDE). J Transl Med 17(1):86
Sevencan NO, Ozkan AE (2019) Associations between neutrophil/lymphocyte ratio, platelet/lymphocyte ratio, albuminuria and uric acid and the estimated glomerular filtration rate in hypertensive patients with chronic kidney disease stages 1–3. Arch Med Sci 15(5):1232–1239
Zhao WM, Tao SM, Liu GL (2020) Neutrophil-to-lymphocyte ratio in relation to the risk of all-cause mortality and cardiovascular events in patients with chronic kidney disease: a systematic review and meta-analysis. Ren Fail 42(1):1059–1066
MacIsaac RJ, Ekinci EI, Premaratne E et al (2015) The chronic kidney disease-epidemiology collaboration (CKD-EPI) equation does not improve the underestimation of glomerular filtration rate (GFR) in people with diabetes and preserved renal function. BMC Nephrol 16(1):198
Eknoyan G, Lameire N, Eckardt K et al (2013) KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney int 3(1):5–14
Bain BJ (2017) Preparation and staining methods for blood and bone marrow films. In: Barbara J. Bain, Imelda Bates, Michael A. Laffan (eds) Dacie and Lewis practical haematology, 12th edn. Elsevier. https://doi.org/10.1016/B978-0-7020-6696-2.00004-7
Rapa SF, Di Iorio BR, Campiglia P, Heidland A, Marzocco S (2019) Inflammation and oxidative stress in chronic kidney disease-potential therapeutic role of minerals, vitamins and plant-derived metabolites. Int J Mol Sci. 21(1):263
Altunoren O, Akkus G, Sezal DT et al (2019) Does neutrophil to lymphocyte ratio really predict chronic kidney disease progression? Int Urol Nephrol 51(1):129–137
Stenvinkel P, Chertow GM, Devarajan P, Levin A, Andreoli SP, Bangalore S, Warady BA (2021) Chronic inflammation in chronic kidney disease progression: role of Nrf2. Kidney Int Rep 6(7):1775–1787
Gungor O, Unal HU, Guclu A et al (2017) IL-33 and ST2 levels in chronic kidney disease: associations with inflammation, vascular abnormalities, cardiovascular events, and survival. PLoS ONE 12(6):e0178939
Akchurin OM, Kaskel F (2015) Update on inflammation in chronic kidney disease. Blood Purif 39(1–3):84–92
Kotani K, Sakane N (2012) White blood cells, neutrophils, and reactive oxygen metabolites among asymptomatic subjects. Int J Prev Med 3(6):428–431
Dzieniszewski J, Jarosz M, Szczygieł B et al (2005) Nutritional status of patients hospitalised in Poland. Eur J Clin Nutr 59(4):552–560
Kuo IC, Huang JC, Wu PY, Chen SC, Chang JM, Chen HC (2017) A low geriatric nutrition risk index is associated with progression to dialysis in patients with chronic kidney disease. Nutrients. 9(11):1228
Terawaki H, Yoshimura K, Hasegawa T et al (2004) Oxidative stress is enhanced in correlation with renal dysfunction: examination with the redox state of albumin. Kidney Int 66(5):1988–1993
Gan W, Guan Q, Hu X et al (2022) The association between platelet-lymphocyte ratio and the risk of all-cause mortality in chronic kidney disease: a systematic review and meta-analysis. Int Urol Nephrol 54(11):2959–2967
Okyay GU, İnal S, Öneç K et al (2013) Neutrophil to lymphocyte ratio in evaluation of inflammation in patients with chronic kidney disease. Ren Fail 35(1):29–36
Kocyigit I, Eroglu E, Unal A, Sipahioglu MH, Tokgoz B, Oymak O, Utas C (2013) Role of neutrophil/lymphocyte ratio in prediction of disease progression in patients with stage-4 chronic kidney disease. J Nephrol 26(2):358–365
Ahbap E, Sakaci T, Kara E et al (2016) Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in evaluation of inflammation in end-stage renal disease. Clin Nephrol 85(4):199–208
Turkmen K, Erdur FM, Ozcicek F et al (2013) Platelet-to-lymphocyte ratio better predicts inflammation than neutrophil-to-lymphocyte ratio in end-stage renal disease patients. Hemodial Int 17(3):391–396
Umeres-Francia GE, Rojas-Fernández MV, Herrera-Añazco P, Benites-Zapata VA (2022) Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio as risk factors for mortality in Peruvian adults with chronic kidney disease. Renal Replacement Therapy 8(1):30
Brito GMC, Fontenele AMM, Carneiro E et al (2021) Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios in nondialysis chronic kidney patients. Int J Inflam 2021:6678960
Valga F, Monzón T, Henriquez F, Antón-Pérez G (2019) Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as biological markers of interest in kidney disease. Nefrología (English Edition) 39(3):243–249
Cravedi P, Remuzzi G (2013) Pathophysiology of proteinuria and its value as an outcome measure in chronic kidney disease. Br J Clin Pharmacol 76(4):516–523
Acknowledgements
We would like to thank nurses, medical officers, and other supporting staff at the Nephrology Clinic, Teaching Hospital Jaffna, for introducing patients into this study and blood and urine sampling from patients.
Funding
This study did not receive any funding.
Author information
Authors and Affiliations
Contributions
KR and BT conceived the idea and design of methodology. FAA, NS, FAH, KR, and BT did the data collection and laboratory investigation. KR, FAA, NS, and FAH analyzed the result. KR and FAA wrote the initial draft. KR and BT critically reviewed and modified the manuscript. All authors read the manuscript, offered feedback, and approved it before submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was performed after getting approval from the Ethics Review Committee of the Faculty of Medicine, University of Jaffna, Sri Lanka (It was approved at its 136th meeting held on 23rd June 2022). All patients provided written informed consent prior to undergoing the necessary procedures.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aneez, F.A., Shariffdeen, N., Haleem, F.A. et al. Correlation between neutrophil to lymphocyte ratio and platelet to lymphocyte ratio with proteinuria in different stages of chronic kidney disease. Egypt J Intern Med 36, 6 (2024). https://doi.org/10.1186/s43162-023-00270-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43162-023-00270-9