Abstract
Immunotherapy medicines (immune checkpoint inhibitors, ICIs) that work directly on the immune system have shown vastly increased survival for people with cancer in phases 2 and 3 clinical studies during the past few years. Nevertheless, ICI treatment (irAEs) may trigger immune-related adverse effects. An underactive thyroid is among the most frequent endocrine irAE, affecting about 40% of individuals who received ICIs. Our review aims to collect and organize the most recent data on immunotherapy-induced thyroid dysfunction in cancer patients, including its prevalence, diagnostic criteria, and treatment options and to summarize those findings in a comprehensive review article. The incidence of irAEs varies depending on the type of cancer and the treatment regimen. Thyroid ultrasound, radioactive uptake scan, and PET CT scan can aid in diagnosing thyroid dysfunction. Thyroid dysfunction treatment necessitates collaboration between specialists in oncology, endocrinology, and primary care in a multidisciplinary team discussion. The prognosis of patients who suffered from thyroid dysfunction while on ICIs treatment is reasonably good. Suboptimal baseline thyroid function was linked with decreased overall survival (OS) among ICI-treated patients, but initiating replacement hormonal therapy after ICI initiation was associated with enhanced OS. More research work is required to identify these links and mechanisms of action.
Similar content being viewed by others
Introduction
Immune checkpoint inhibitors (ICIs) stimulate (switch on) the immune system to recognize and attack cancer cells. However, these medications have been linked to various autoimmune disorders, such as thyroid dysfunction [1]. Thyroid dysfunction induced by ICIs depends on the type of immunotherapy administered. According to an intriguing 2022 meta-analysis by Muir et al., programmed cell death protein 1 (PD-1) inhibitors (nivolumab and pembrolizumab) caused 3.2% of hyperthyroidism, while combo immunotherapy with ipilimumab caused 8.0% [2]. Combination therapy caused 13.2% more hypothyroidism than PDL-1 inhibitors alone. PD-1 inhibitors alone took 70 days, and combined therapy took 63 days to onset [3].
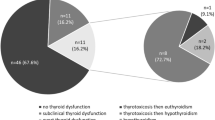
Haanen et al. (2017) found an incidence of 1–5 to 10% for individuals receiving 3 mg/kg and 10 mg/kg ipilimumab [4]. In a more recent retrospective investigation by Girimonte et al. (2022), 29.6% (53 out of 179) of metastatic cancer patients taking ICIs developed hypothyroidism, with 44 of those instances experiencing transient thyrotoxicosis followed by hypothyroidism [5]. While treating cancer with anti-PD-1 or anti-PD-L1, the risk of thyroid abnormalities is 5–10%, with a higher prevalence in combination therapy patients.
In recent years, research has aimed to understand better the endocrine toxicities arising from ICI therapy and the side effects patients can expect. These events rarely exceed 20% severity [4]. Several studies on immunotherapy-induced endocrinopathy have found these results. Lecoq et al. (2022) thoroughly reviewed the relevant literature and analyzed their clinical observations from treating 80 patients. Their findings showed that thyroiditis and hypothyroidism were the most common endocrinopathies reported with ICI treatment, particularly when using anti-PD-1 medication. Patients taking anti-CTLA-4 and anti-PD-1 medicines were at a higher risk of developing endocrinopathies [6].
Similarly, Storm et al. (2022) investigated the real-world safety profile of ICIs in younger and older participants between September 2016 and September 2019. The study enrolled 217 patients who received ICIs and found no statistically significant difference in the cumulative incidence of immunotherapy-related toxicities between the two age groups, although thyroid gland problems occurred in 20.3% of patients. These results suggest that older adults are less likely to experience immune-related adverse medication responses from ICIs [7].
In another study, among 24 cancer patients receiving ICI, Patrizio et al. (2022) observed that using these medications raised the incidence of thyroid disease, including subclinical illness, with the latter being more common in women than in males [8]. Degtiareva et al. (2022) also found that immune-related toxicity was more common with combination ICIs than with monotherapy (70.6% vs. 23.6%) among Russian patients in a retrospective study [9]. Additionally, Ochenduszko et al. (2022) found that among 36 patients with advanced melanoma receiving either nivolumab or pembrolizumab monotherapy, 5 of them (13.9%) developed hypothyroidism [10].
Mechanism and risk factors
The exact mechanisms underlying immunotherapy-induced thyroid dysfunction remain unclear. ICIs are typically harmless, but some evidence suggests they may provoke an autoimmune reaction in the thyroid gland by disturbing the delicate balance of immune cells or by producing cross-reactive cancer cell antigens. ICIs may also change thyroid-related gene expression, causing hypothyroidism [11]. Several researchers have investigated possible risk factors for endocrinopathies in patients receiving ICIs to better understand this phenomenon's origins. In 2022, Amara et al. examined the causes of immune-related thyroid dysfunction during ICIs treatment [12]. The study found that among cancer patients taking PD-1/PD-L1 inhibitors, a history of smoking, hypertension, or opioid use was all associated with adverse events involving the thyroid [12]. Another study found that immunotherapy-related thyroid dysfunction may be linked to a hereditary predisposition to autoimmune thyroid disease [13].
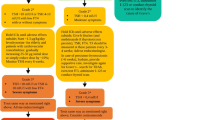
Screening and monitoring for thyroid dysfunction
It is essential to regularly check for thyroid dysfunction in patients receiving ICIs, as early detection and treatment can prevent more serious consequences. While there are various screening recommendations, most suggest baseline thyroid function testing and regular monitoring of thyroid-stimulating hormone (TSH) and free thyroxine (FT4) levels both before and after ICI therapy [14]. The American Thyroid Association (ATA) recommends monitoring thyroid dysfunction symptoms/signs and checking TSH and FT4 levels at least every 4 to 8 weeks while on ICI treatment therapy and every 3 to 6 months afterward [3].
Monitoring thyroglobulin (Tg) and anti-TgAb can assist in diagnosing thyroid autoimmune reactions and track a patient’s response to thyroid dysfunction treatment [15]. However, the frequency of checks should be determined by the patient’s status and risk factors. It is important to note that patients receiving ICIs may develop thyroid dysfunction at any point during or after treatment. Thus, thorough monitoring is crucial throughout the treatment [16]. The most recent recommendations from the European Society for Medical Oncology (ESMO) suggest measuring TSH and FT4 levels periodically before starting immunotherapy and before each cycle at least monthly intervals [17]. Recent studies have also examined the clinical trajectories and risk factors for enduring immune-related toxicities in cancer patients receiving anti-PD-1, anti-PD-L1, and/or combination with anti-CTLA-4 therapy. Chieng et al. (2022) studied 66 patients and followed them for a median of 15.7 months [18]. The study found that the average duration of thyroid dysfunction prior to diagnosis was 1.8 months. The study also found that persistent thyroid disorder was linked to positive thyroperoxidase antibodies (TPOAb) and/or thyroglobulin antibodies (TgAb) status at the onset. Interestingly, the study also found that patients who developed endocrinopathies had a longer median survival [18]. This suggests that early screening is crucial given the large median time to onset of endocrinopathies, and that the screening and follow-up strategy for endocrine irAEs should be tailored to each endocrinopathy’s clinical history [18].
Clinical manifestations
Immunotherapy-induced thyroid dysfunction can cause fatigue, weight gain, heart rate changes, and thyroid hormone abnormalities [19]. Hence, ICI patients must be monitored regularly. Latif et al. (2022) examined thyroid dysfunction in advanced cancer patients treated with ICIs at two United Arab Emirates (UAE) tertiary cancer centers from November 2015 to January 2019 [20]. Of the 43 people who received ICI, 44% or more had some form of thyroid malfunction, either hypothyroidism (57%), hyperthyroidism, or subclinical hypothyroidism (21%). ICI caused most thyroid dysfunction after 6 weeks [20].
Role of thyroid ultrasound to detect and ICIs-induced thyroid dysfunction
Thyroid ultrasonography can detect thyroid gland changes in immunotherapy patients on immune checkpoint inhibitors (ICIs). Thyroid nodules, a larger or smaller gland, may suggest hyperthyroidism or hypothyroidism. Ultrasound can detect autoimmune disorders such as diffuse or localized thyroiditis [21]. Furthermore, thyroid ultrasonography can also be utilized to monitor the effectiveness of treatment in patients with thyroid dysfunction who are receiving ICIs. For instance, ultrasonography can be used to evaluate the effectiveness of antithyroid medication in treating hyperthyroidism by determining whether the size and shape of the thyroid gland have returned to normal [21]. However, thyroid ultrasound results should be reviewed with other diagnostic methods, such as blood tests and clinical examinations, and analyzed by a skilled physician for proper and accurate diagnosis. It is crucial to remember that thyroid ultrasound is an auxiliary tool.
Role of thyroid radioactive uptake scan with ICIs
A radioactive thyroid uptake scan can play a crucial role in assessing patients who have developed hyperthyroidism or hypothyroidism in immunotherapy. This diagnostic method can pinpoint thyroid dysfunction’s etiology, such as a toxic nodule or Graves’ disease. The scan can also reveal whether the thyroid is hyperactive or underactive. The test can also detect autoimmune responses by measuring thyroid gland radioactivity uptake. For example, reduced radioactive material uptake in patients with autoimmune thyroiditis may indicate autoimmune alterations in the thyroid gland [22].
Role of inflammatory markers and biomarker on thyroid disease-related ICIs
Inflammatory indicators such as cytokines, TNF-alpha, IL-6, and IFN-gamma, all of which can damage tissue through the recruitment, activation, and invasion of inflammatory cells into the thyroid gland, have been linked recently to thyroid dysfunction [23]. In terms of biomarkers, a recent trial conducted in 2022 by Amara et al. found that 9 out of 20 patients with lung cancer who received immunotherapy developed irAEs, including thyroid dysfunction [12]. The study discovered that the CD4/CD8 ratio consistently dropped by 30–40% just before the onset of ICI-induced toxicities. The study suggests that biomarkers such as the serum CD4/CD8 ratio may be crucial for early management in cases of irAEs [12]. These findings highlight the importance of monitoring inflammatory markers and biomarkers in managing and monitoring patients with thyroid dysfunction related to ICIs.
Role of PET CT scan
Tatar et al. studied [18F]-fluoro-2-deoxy-D-glucose (F-FDG) PET/CT for detecting immune-related adverse events (irAEs) in immune checkpoint inhibitor patients in 2022 (ICIs). The study enrolled 46 patients with diverse forms of advanced cancer, and their treatment responses were monitored using PET/CT scanning [24]. The study found that among the most frequently observed adverse effects, colitis (28.1%) stood out, while among the least often observed adverse effects, thyroiditis and myositis/arthritis (13.0%) affected only about six individuals. The study also found that the appearance of irAEs on PET/CT occurred a median of 4.3 months after the start of immunotherapy. The study’s results suggest that F-FDG PET/CT is an important aspect of cancer immunotherapy, as it can detect serious irAEs during diagnosis and follow-up. irAEs were detected on PET/CT scans in more than 50% of the trial patients [24]. The study concludes that implementing F-FDG PET/CT scans in the management and follow-up of patients receiving ICIs is essential for the early detection and management of irAEs [24].
Treatment
Treating thyroid dysfunction caused by immunotherapy requires a multidisciplinary approach [21]. Patients with thyroid dysfunction should delay immunotherapy until their symptoms improve, according to the latest ESMO guidelines. Antithyroid drugs, beta-blockers, and even radioactive iodine can be used in protocols to treat hyperthyroidism. Levothyroxine is usually used to cure hypothyroidism. Subclinical hypothyroidism does not preclude thyroid hormone replacement therapy for patients with fatigue and weight gain symptoms. Propranolol and atenolol are also used in the treatment of hyperthyroidism. Such medicines provide long-term advantages in controlling symptoms and improving tolerability to ICIs [4].
An American Association of Clinical Endocrinology Disease review article provides healthcare professionals with a helpful method for managing patients who develop endocrinopathies due to immunotherapy. These agents frequently affect the thyroid and pituitary glands, and the authors conduct a literature search and review methods for their diagnosis and treatment. The researchers emphasize the need for all healthcare professionals caring for these cancer patients to have a high index of clinical suspicion and stress the significance of multidisciplinary team discussion in managing patients receiving immunotherapy and exhibiting endocrinopathies [21].
In terms of levothyroxine dosing for patients developing de novo hypothyroidism, particularly hypophysitis, a recent retrospective study in 2022 by Kristan et al. suggests a more conservative approach, such as starting at 0.9–1.2 mcg/kg and monitoring thyroid function tests (TFTs) every 4 weeks and spacing out TFT surveillance to every 12 weeks after [25].
Prognosis
A study conducted by von Itzstein et al. in 2022 found that suboptimal baseline thyroid function was associated with decreased overall survival (OS) among patients treated with ICIs. However, initiating replacement hormonal therapy with levothyroxine after ICI initiation was associated with enhanced OS [26]. The study suggests that existing thyroid problems may predict worse outcomes for individuals undergoing ICI therapy [26]. A 2022 study by Trudu et al. found that patients treated with ICIs for spread lung cancer had a median progression-free survival of 9.5 months [27]. Thyroid dysfunction was the most common immune-related adverse event (30.7%).
Those who had immune-related adverse events (irAEs) had a significantly longer median progression-free survival (PFS) [28]. Improvements in overall survival were seen (HR 0.63, 95% CI 0.43–0.89) [28]. irAEs in immunotherapy-treated cancer patients may indicate better treatment efficacy and survival [28]. Zheng et al. found that 40% of anti-PD-1-treated hepatocellular carcinoma (HCC) patients in his trial had hypothyroidism; however, this did not impair survival [29]. These relationships and mechanisms need further study.
Case reports
The following table presents immunotherapy-thyroid disease case reports during 2022–2023 (Table 1).
Conclusion
Thyroid dysfunction, which may arise as a side effect of immunotherapy, should be managed cautiously by a multidisciplinary group of oncologists, endocrinologists, and primary care physicians, as highlighted in the article. Treatment options — subject to symptoms — may include using beta-blockers, antithyroid drugs, radioactive iodine, or hormone replacement therapy after proper counseling with patients. The use of thyroid ultrasonography, radioactive uptake scan, inflammatory markers, biomarkers, and PET-CT scans can aid in diagnosing and monitoring thyroid dysfunction. The initiation of replacement hormonal therapy with levothyroxine after ICI initiation was associated with enhanced overall survival. The emergence of irAEs in cancer patients treated with immunotherapy may indicate greater treatment success and overall survival; however, further research is needed to validate these connections and mechanisms of action. Having a high index of clinical suspicion and a multidisciplinary team discussion in managing patients receiving immunotherapy and exhibiting endocrinopathies is essential.
Availability of data and materials
Not applicable.
Abbreviations
- ALK:
-
Anaplastic lymphoma kinase
- anti-CTLA-4:
-
Anti-cytotoxic T-lymphocyte-associated antigen-4
- anti-PD-L1:
-
Anti-programmed cell death 1 ligand
- CAA:
-
Cerebral amyloid angiopathy
- CAR-T:
-
Chimeric antigen receptor T cell
- CSF:
-
Cerebrospinal fluid
- EGFR:
-
Epidermal growth factor receptor
- ESMO:
-
European Society for Medical Oncology
- F-FDG:
-
Fluoro-2-deoxy-D-glucose
- FT4:
-
Free thyroxine
- HLA-DR4:
-
Human leukocyte antigen DR4
- ICIs:
-
Immune checkpoint inhibitors
- IFN-gamma:
-
Interferon‐gamma
- IGF-1R:
-
The insulin-like growth factor 1 receptor
- IL-6:
-
Interleukin 6
- irAEs:
-
Immune-related adverse event
- MRI:
-
Magnetic resonance imaging
- N/A:
-
Not available
- NSCLC:
-
Non-small cell lung carcinoma
- OS:
-
Overall survival
- PD-1:
-
Anti-programmed cell death 1
- PD-L1:
-
Programmed death-ligand 1
- PET/CT:
-
Positron emission tomography-computed tomography
- PFS:
-
Progression-free survival
- TFTs:
-
Thyroid function tests
- Tg:
-
Thyroglobulin
- TgAb:
-
Anti-thyroglobulin antibodies
- TNF-alpha:
-
Tumor necrosis factor-alpha
- TSH:
-
Thyroid-stimulating hormone
References
Gun SY, Lee SWL, Sieow JL, Wong SC (2019) Targeting immune cells for cancer therapy. Redox Biol 25:101174. https://doi.org/10.1016/j.redox.2019.101174
Barroso-Sousa R, Barry WT, Garrido-Castro AC, Hodi FS, Min L, Krop IE, Tolaney SM (2018) Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta-analysis. JAMA Oncol 4:173–182. https://doi.org/10.1001/jamaoncol.2017.3064
El Sabbagh R, Azar NS, Eid AA, Azar ST (2020) Thyroid dysfunctions due to immune checkpoint inhibitors: a review. Int J Gen Med 13:1003–1009. https://doi.org/10.2147/IJGM.S261433
Haanen J, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, Jordan K, Committee EG (2017) Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 28:iv119–iv142. https://doi.org/10.1093/annonc/mdx225
Girimonte D, Basile D, Daffina MG, Dottore A, Ventura M, Cortese C, Liguori A, Lacaria M, Sacchetta S, Garigliano D, Siciliano AM, Alessandro D, Anania M, Prantera T (2022) AIOM abstract Tumori Journal 108:1–194. https://doi.org/10.1177/03008916221114500
Lecoq AL, Becquemont L, Chanson P: Poster abstracts. fundamental & clinical pharmacology. 2022, 36:92-169. https://doi.org/10.1111/fcp.12789
Storm BN, AbedianKalkhoran H, Wilms EB, Brocken P, Codrington H, Houtsma D, Portielje JEA, de Glas N, van der Ziel D, van den Bos F, Visser LE (2022) Real-life safety of PD-1 and PD-L1 inhibitors in older patients with cancer: an observational study. J Geriatr Oncol 13:997–1002. https://doi.org/10.1016/j.jgo.2022.05.013
Patrizio A, Fallahi P, Antonelli A, Ferrari SM (2023) Immune checkpoint inhibitor-induced thyroid disorders: a single center experience. Curr Pharm Des 29:295–299. https://doi.org/10.2174/1381612828666220518151509
Degtiareva E, Protsenko S, Iyevleva A, Imyanitov E (2022) Real-world data on the incidence of immune-related adverse events associated with anti-PD-1/PD-L1 treatment in Russia. Problems in oncology 68:188–199. https://doi.org/10.37469/0507-3758-2022-68-2-188-199
Ochenduszko S, García J, Juan-Fita MJ, Gonzalez-Barrallo I, Herrero Colomina J, Diaz Beveridge R, Ros Martinez S, Sanchez Lafuente B, Cunquero Tomas AJ, Berrocal A: Real-world study on characteristics of advanced melanoma patients with sustained complete response (CR) after elective 1st line anti-programmed death-1 (anti-PD1) monotherapy discontinuation. American Society of Clinical Oncology; 2022.
Tison A, Garaud S, Chiche L, Cornec D, Kostine M (2022) Immune-checkpoint inhibitor use in patients with cancer and pre-existing autoimmune diseases. Nat Rev Rheumatol 18:641–656. https://doi.org/10.1038/s41584-022-00841-0
Amara S, Muzaffar M, Namireddy P, Yang LV (2022) Peripheral blood t cell responses to immunotherapy related adverse events in metastatic non-small cell lung cancer. J Clin Oncol 40:e21047–e21047. https://doi.org/10.1200/JCO.2022.40.16_suppl.e21047
Muir CA, Tsang VHM, Menzies AM, Clifton-Bligh RJ (2022) Immune related adverse events of the thyroid - a narrative review. Front Endocrinol (Lausanne) 13:886930. https://doi.org/10.3389/fendo.2022.886930
Yonezaki K, Kobayashi T, Imachi H, Yoshimoto T, Kikuchi F, Fukunaga K, Sato S, Ibata T, Yamaji N, Lyu J, Dong T, Murao K (2018) Combination therapy of ipilimumab and nivolumab induced thyroid storm in a patient with Hashimoto’s disease and diabetes mellitus: a case report. J Med Case Rep 12:171. https://doi.org/10.1186/s13256-018-1708-x
Baric A, Brcic L, Gracan S, Skrabic V, Brekalo M, Simunac M, Lovric VT, Anic I, Barbalic M, Zemunik T, Punda A, BoraskaPerica V (2019) Thyroglobulin antibodies are associated with symptom burden in patients with hashimoto’s thyroiditis: a cross-sectional study. Immunol Invest 48:198–209. https://doi.org/10.1080/08820139.2018.1529040
Paschou SA, Stefanaki K, Psaltopoulou T, Liontos M, Koutsoukos K, Zagouri F, Lambrinoudaki I, Dimopoulos MA (2021) How we treat endocrine complications of immune checkpoint inhibitors. ESMO Open 6:100011. https://doi.org/10.1016/j.esmoop.2020.100011
Husebye ES, Castinetti F, Criseno S, Curigliano G, Decallonne B, Fleseriu M, Higham CE, Lupi I, Paschou SA, Toth M, van der Kooij M, Dekkers OM (2022) Endocrine-related adverse conditions in patients receiving immune checkpoint inhibition: an ESE clinical practice guideline. Eur J Endocrinol 187:G1–G21. https://doi.org/10.1530/EJE-22-0689
Chieng JHL, Htet ZW, Zhao JJ, Tai ES, Tay SH, Huang Y, Wong A, Yang SP (2022) Clinical presentation of immune-related endocrine adverse events during immune checkpoint inhibitor treatment. Cancers (Basel) 14:2687. https://doi.org/10.3390/cancers14112687
Hattersley R, Nana M, Lansdown AJ (2021) Endocrine complications of immunotherapies: a review. Clin Med (Lond) 21:e212–e222. https://doi.org/10.7861/clinmed.2020-0827
Latif MF, Abdelgadir E, Omara M, Rashid F, Tirmazy SH, Khan F, El Khoury M, Bashier A, Alawadi F, Das K, Kumar S, Basit AQ, El-Shourbagy D, Hamza D, Azam F (2022) Immune checkpoint inhibitors-induced thyroid dysfunction in patients with advanced malignancies. Middle East Journal of Cancer. 13:616–623. https://doi.org/10.30476/mejc.2022.89620.1538
Yuen KCJ, Samson SL, Bancos I, Gosmanov AR, Jasim S, Fecher LA, Weber JS (2022) American Association of Clinical Endocrinology Disease State Clinical Review: evaluation and management of immune checkpoint inhibitor-mediated endocrinopathies: a practical case-based clinical approach. Endocr Pract 28:719–731. https://doi.org/10.1016/j.eprac.2022.04.010
Ross DS, Burch HB, Cooper DS, Greenlee MC, Laurberg P, Maia AL, Rivkees SA, Samuels M, Sosa JA, Stan MN, Walter MA (2016) 2016 American Thyroid Association Guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid 26:1343–1421. https://doi.org/10.1089/thy.2016.0229
Chalan P, Di Dalmazi G, Pani F, De Remigis A, Corsello A, Caturegli P (2018) Thyroid dysfunctions secondary to cancer immunotherapy. J Endocrinol Invest 41:625–638. https://doi.org/10.1007/s40618-017-0778-8
Tatar G, Alcin G, SengulSamanci N, ErolFenercioglu O, Beyhan E, Cermik TF (2022) Diagnostic impact of (18)F-FDG PET/CT imaging on the detection of immune-related adverse events in patients treated with immunotherapy. Clin Transl Oncol 24:1903–1913. https://doi.org/10.1007/s12094-022-02840-9
Kristan MM, Toro-Tobon D, Francis N, Desale S, Bikas A, Jonklaas J, Goyal RM: Immunotherapy-associated hypothyroidism: comparison of the pre-existing with de-novo hypothyroidism. Front Endocrinol (Lausanne). 2022, 13:798253. 10.3389/fendo.2022.798253
von Itzstein MS, Gonugunta AS, Wang Y, Sheffield T, Lu R, Ali S, Fattah FJ, Xie D, Cai J, Xie Y, Gerber DE (2022) Divergent prognostic effects of pre-existing and treatment-emergent thyroid dysfunction in patients treated with immune checkpoint inhibitors. Cancer Immunol Immunother 71:2169–2181. https://doi.org/10.1007/s00262-022-03151-2
Trudu L, Guaitoli G, Bertolini F, Maur M, Santini C, Papapietro VR, Talerico S, Natalizio S, Isca C, Dominici M, Barbieri F (2022) Thyroid function impairment after chemo-immunotherapy for advanced NSCLC: a single institutional retrospective report. Immunotherapy 14:675–682. https://doi.org/10.2217/imt-2021-0165
Prather LL, Ali A, Wang H, Du W, Chen N, Sheth A, Sandulache V, Sabichi AL, Kemnade JO, Wang DY (2022) Immune-related adverse events and immunotherapy efficacy in patients with cancer: a retrospective study. J Clin Oncol 40:2654–2654. https://doi.org/10.1200/JCO.2022.40.16_suppl.2654
Zheng X, Xiao H, Long J, Wei Q, Liu L, Zan L, Ren W (2022) Dynamic follow-up of the effects of programmed death 1 inhibitor treatment on thyroid function and sonographic features in patients with hepatocellular carcinoma. Endocr Connect. 11:e220065
Marinides Z, Gower C, Kidd C, Werner J: Rapidly progressive dementia from treatment with a novel IGF-1R inhibitor (P8–1.005). AAN Enterprises; 2022.
Vilaca M, Silva C, Estevinho F, Magalhaes H: Immunotherapy in stage IV non-small cell lung cancer in a patient with Grave’s disease: safety and biomarkers of response. BMJ Case Rep. 2022, 15. https://doi.org/10.1136/bcr-2021-245632
Duminuco A, Cupri A, Milone GA, Marcantoni C, Leotta S, Esposito B, Garibaldi B, Chiarenza A, Milone G: Previous therapy with immune checkpoint inhibitor as a cause of hypothyroidism, myositis, and renal insufficiency in a candidate for allogeneic hematopoietic transplantation. Transpl Immunol. 2022, 75:101705. 10.1016/j.trim.2022.101705
Najjar W, Yu J: Audiologic demonstration of ototoxicity from teprotumumab treatment in a patient with thyroid eye disease. OTO Open. 2022, 6:2473974X221097097. https://doi.org/10.1177/2473974X221097097
Kataoka S, Matsuno K, Sugano K, Takahashi K: Thyroid storm induced by combined nivolumab and ipilimumab immunotherapy in advanced non-small cell lung cancer. BMJ Case Rep. 2022, 15. https://doi.org/10.1136/bcr-2022-250696
de Filette JMK, Andre S, De Mey L, Aspeslagh S, Karmali R, Van der Auwera BJ, Bravenboer B (2022) Durvalumab-induced thyroiditis in a patient with non-small cell lung carcinoma: a case report and review of pathogenic mechanisms. BMC Endocr Disord 22:291. https://doi.org/10.1186/s12902-022-01190-5
Bao S, Jiang X (2022) Anti-PD-1 immune checkpoint inhibitor inducing endocrine toxicity in a patient with advanced lung cancer: a case report and literature review. Exp Ther Med 24:681. https://doi.org/10.3892/etm.2022.11617
Chen P, Xia Y, Lei W, Zhong S, Jiang H, Ren L, Qian W, Liu H: Case report: Hashimoto’s thyroiditis after CD19 chimeric antigen receptor T-cell therapy. Front Immunol. 2022, 13:995496. 10.3389/fimmu.2022.995496
Braga S, Barreto J, Torgal A, Pereira J, Leão A, Gonçalves N, Araújo L (2022) T170 Three cases of endocrine immune-related adverse events caused by immune checkpoint inhibitor therapy. Clin Chim Acta 530:S136. https://doi.org/10.1016/j.cca.2022.04.649
Acknowledgements
Not applicable
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
MN and BB wrote the main manuscript. MN and HA edited and reviewed. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Baraka, B., Abosheaishaa, H. & Nassar, M. Immunotherapy-induced thyroid dysfunction: an updated review. Egypt J Intern Med 35, 48 (2023). https://doi.org/10.1186/s43162-023-00210-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43162-023-00210-7