Abstract
Background
Malnutrition is highly prevalent in chronic kidney disease (CKD). This study is conducted to find out the prevalence of malnutrition and its association with inflammation in patients with CKD stages 3–5.
Method
This is a hospital-based cross-sectional study conducted at the Sri Ram Murti Smarak Institute of Medical Science, Uttar Pradesh, India. CKD stages 3–5 patients were included. The nutritional status was assessed by subjective global assessment (SGA). Anthropometric and biochemical measurements were also checked at the time of enrollment.
Results
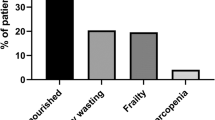
A total of 213/354 (60.2%) of patients were malnourished. The triceps skinfold thickness (TSFT) was 8.2 ± 1.2 mm and 10.9 ± 1.2 mm in the malnourished and well-nourished groups, respectively; p < 0.001. TSFT was ≤ 10 mm in 91.1% of patients with malnutrition. Mid-arm muscle circumference (MAMC) was 21.3 ± 2.2 cm and 24 ± 2.9 cm, and the body mass index (BMI) was 19.8 ± 1.5 and 22.5 ± 1.4 kg/m2 in the malnourished and well-nourished group, respectively; p < 0.001. A significant direct correlation between BMI, TSFT, MAMC, and eGFR was noted. Median eGFR was 11.9 ml/min/1.73 m2 in the malnourished compared to 24.2 ml/min/1.73 m2 in the well-nourished group; p < 0.001. Serum ferritin level was 246.77 ± 18.24 mg/L in the malnourished group, higher than the well-nourished group at 237.23 ± 16.13 mg/L; p < 0.001. CRP was elevated (> 0.6 mg/dl) in 53.5% patients with malnutrition; p 0.003.
Conclusions
Malnutrition is highly prevalent in the CKD population. The incidence increases as the eGFR decrease. TSFT ≤ 10 mm compares favorably with SGA in detecting malnutrition (sensitivity > 90%), and BMI > 20 kg/m2 compares favorably with SGA in ruling out malnutrition (specificity 97%). Malnutrition and inflammation often coexist. Early detection and appropriate management are crucial.
Similar content being viewed by others
Introduction
Malnutrition is frequent in patients with chronic kidney disease, with prevalence varying from 28 to 65% [1,2,3,4,5]. It affects the quality of life and is associated with increased mortality and morbidity [6,7,8,9]. Causes of malnutrition in chronic kidney disease (CKD) patients include reduced food intake due to the effect of uremia, reduced absorption of nutrients from the oedematous gut, metabolic acidosis, increased protein loss during dialysis especially peritoneal dialysis, inflammation, oxidative stress, carbonyl stress, and hormonal disorders [10,11,12,13]. There is no single ideal or well-established laboratory method for the diagnosis of malnutrition in patients with CKD. In 1987, Detzky AS et al. [14] described a special methodology, named subjective global assessment (SGA) for the assessment of nutritional status. It is an easy and simple method of assessing nutritional status and does not require any additional laboratory testing [15, 16]. It has been found to strongly correlate with other subjective and objective measures of nutrition in patients with pre-dialysis CKD [17] and patients on hemodialysis [18] or peritoneal dialysis [19]. Malnutrition was found in 46% of CKD patients studied using the SGA criteria by Oladele CO et al. [20] A higher prevalence of 54.8% was observed in a similar study by Liman et al. [21], using the SGA. There is a paucity of data on the nutritional status of chronic kidney disease stages 3–5 in patients from northern India, necessitating this study.
Material and methods
This is a hospital-based cross-sectional study conducted in the Department of Nephrology, Sri Ram Murti Smarak Institute of Medical Science, Uttar Pradesh, India, from 1st October 2018 to 30th May 2020. Patients with chronic kidney disease stages 3–5 were included. Patients on dialysis, patients with nephrotic range proteinuria, and patients on steroids were excluded. CKD was defined as an estimated glomerular filtration rate < 60 ml/min/m2 for more than 3 months. The estimated glomerular filtration rate (eGFR) was calculated using creatinine-based CKD-EPI (chronic kidney disease epidemiology collaboration formula) 2009. The nutrition status was assessed by subjective global assessment (SGA). SGA was performed by one observer for all the patients, and this was based on medical history and clinical examination. Medical history included an assessment of weight and weight change, dietary intake, gastrointestinal symptoms, and the patient’s functional capacity related to nutritional status. Physical examination focussed on loss of subcutaneous fat and muscle wasting, the presence of edema, and ascites. Patients were classified into class A (well-nourished), B (mildly to moderately malnourished), and C (severely malnourished) depending on the results of medical history and physical examination. Anthropometric and biochemical measurements were also taken at the time of enrollment. These anthropometric variables can be measured by different methods including dual-energy X-ray absorptiometry or bioelectrical impedance analysis; however, conventional anthropometric measures in the form of body mass index (BMI), triceps skinfold thickness (TSFT), and mid-arm muscle circumference (MAMC) were used owing to financial constraints and nonavailability of these modalities at the study center. TSFT was measured in the midline of the posterior aspect of the arm, over the triceps muscle at the level, 1 cm proximal to the halfway between the acromion process and the olecranon process with the elbow flexed at 90°. A vertical fold of skin and subcutaneous tissue were picked gently with the left thumb, and the tips of Harpenden caliper were applied perpendicular to the skinfold at the marked level. MAMC was measured using a measuring tape, midway between the olecranon process and the acromion. The left upper limb was used for the calculation of TSFT and MAMC in all the patients. Height was measured using a stadiometer, and weight was measured using a standard weighing scale to the nearest 0.1 kg. The study was approved by the hospital ethics committee.
Statistical analysis
Continuous variables are presented as mean ± SD. Nonparametric data are expressed as median and range. The chi-square test is used to compare nonparametric categorical variables. Mann-Whitney U-test is used to compare nonparametric continuous variables. Statistics are done using IBM SPSS statistics for Windows version 25.
Results
Out of 354 patients included, 243 (68.6%), 47(13.3%), and 64 (18.1%) belonged to CKD stage 5, stage 4, and stage 3, respectively. Two-hundred thirteen (60.2%) were malnourished as per SGA. A total of 168/213 (78.9%) belonged to stage 5 CKD. Stage 3 and stage 4 CKD accounted for 23/213 (10.8%) and 22/213 (10.3%) of all malnourished cases, respectively. Out of 213 malnourished patients, 118 (55.4%) were mild to moderately malnourished (class B), and 95 (44.6%) were severely malnourished (class C). Men and women accounted for 242/354 (68.4%) and 112/354 (31.6%), respectively. The mean age was 51.68 ± 5.21 years. Diabetes mellitus was the most common cause of CKD followed by hypertension and chronic glomerulonephritis accounting for 36.2%, 22%, and 19.5%, respectively. The male-to-female ratio in the malnourished group was approximately 2:1 (141:72); p-value 0.28. The mean age in the malnourished and well-nourished group was 51.5 ± 5.2 years and 51.9 ± 5.3 years, respectively; p-value 0.95. The triceps skinfold thickness (TSFT) was 8.2 ± 1.2 mm and 10.9 ± 1.2 mm in the malnourished and well-nourished groups, respectively; p-value < 0.001. TFST was ≤ 10 mm in 194/213 malnourished patients corresponding to sensitivity of 91.1% in detecting malnutrition. There was a significant positive correlation between TSFT and eGFR; p-value < 0.001. Mid-arm muscle circumference (MAMC) was 21.3 ± 2.2 cm in the malnourished group versus 24 ± 2.9 cm in the well-nourished group; p-value < 0.001. Out of 161 patients with MAMC ≤ 22 cm, 126 (78.3%) were malnourished, and 35(21.7%) were well-nourished; p-value < 0.001. There was a significant positive correlation between MAMC and eGFR; p-value −0.005. The body mass index (BMI) in the malnourished and well-nourished group was 19.8 ± 1.5 and 22.5 ± 1.4 kg/m2, respectively; p-value < 0.001. Out of 141 well-nourished patients, 137 (97.2%) had BMI > 20 kg/m2 corresponding to specificity of 97.2% in ruling out malnutrition. There was a significant direct correlation between BMI and eGFR; p-value 0.013. Serum triglyceride levels in the malnourished and well-nourished groups were 149.6 ± 15.3 and 138.01 ± 17.2, respectively; p-value < 0.001. Median eGFR in the malnourished group was 11.9 ml/min/1.73 m2 versus 24.2 ml/min/1.73 m2 in the well-nourished group; p-value < 0.001. Serum ferritin level was 246.77 ± 18.24 mg/L and 237.23 ± 16.13 mg/L, respectively, in the malnourished and well-nourished group; p-value < 0.001. Serum albumin in the malnourished and well-nourished groups was 3.03 ± 0.64 mg/dl and 3.50 ± 0.56 mg/dl, respectively; p-value < 0.001. Hypoalbuminemia (serum albumin < 3.5 g/dl) was present in 153/213 (71.8%) of patients with malnutrition; p-value < 0.001. High sensitivity CRP was elevated (> 0.6 mg/dl) in 114/213 (53.5%) patients with malnutrition and 53/141 (37.6%) of well-nourished patients; p-value 0.003. Hemoglobin in the malnourished group and the well-nourished group was 7.74 ± 0.95 mg/dl and 8.84 ± 0.95 mg/dl, respectively; p-value < 0.001. Total protein in the malnourished group versus the well-nourished group was 4.8 ± 0.7 mg/dl and 5.0 ± 0.7 mg/dl, respectively; p-value −0.005. In patients with eGFR ≤ 30 ml/min/1.73 m2, 190/290 (65.5%) were malnourished, whereas in patients with eGFR > 30 ml/min/1.73 m2, only 23/41 (36%) patients were malnourished. In patients with eGFR > 30 ml/min/1.73m2, the difference in serum ferritin between the malnourished and well-nourished groups did not reach statistical significance. Baseline characteristics and the results are summarized in Tables 1, 2, 3, 4, and 5
Discussion
Malnutrition in CKD is not entirely explained by reduced nutritional intake. A delicate interplay of multiple factors, including hormonal imbalances, decreased appetite and food intake, inflammation, increased catabolism, nutrient losses in dialysate, and metabolic derangements, predispose chronic kidney disease patients to malnutrition. There is a paucity of data regarding the prevalence of malnutrition in patients with chronic kidney disease in India. This study highlights the prevalence of malnutrition in CKD stages 3–5 patients from a single center in North India. In our study, out of 354 patients, 213 (60.2%) were malnourished as per SGA criteria. Out of 213 malnourished patients, 118 (55.4%) were mild to moderately malnourished (class B), and 95 (44.6%) were severely malnourished (class C). This is similar to the previously reported prevalence of 28 to 65% [1,2,3,4,5]. A prevalence of 65% was shown by a study from eastern India by Prakash et al. [5] In our study, there was a male predominance in the malnourished group, with a male:female ratio of 141:72 (66.2% vs 33.8%). A similar finding was reported by Prakash et al. [5] who observed that 71% of malnourished CKD patients were men. A study by Peter Stenvinkel et al. [7] also showed male predominance with 61% of patients with CKD and malnutrition being men. This could be because men are more likely to seek medical attention than women. However, the role of hormonal factors could not be ruled out. In our study, there was no statistical difference in age between the patients with malnutrition compared to those who were well-nourished. These findings were similar to the study done by Jai Prakash et al. [5] Diabetes mellitus was the most common cause of chronic kidney disease in our study followed by hypertension, chronic nondiabetic glomerulonephritis, and chronic tubulointerstitial nephritis. Similar results were shown by Kalantar-Zadeh et al. [22] The triceps skinfold thickness (TSFT) was significantly lower in the malnourished compared to the well-nourished groups (8.2 ± 1.2 mm and 10.9 ± 1.2 mm, respectively. TFST was ≤ 10 mm in 91.1% patients with malnutrition and in 16.3% well-nourished patients; p-value < 0.001. This highlights the high sensitivity of TSAT ≤ 10 mm in detecting malnutrition. These findings were similar to Lawson J. A. et al. [1] and by Jai Prakash et al. [5] Mid-arm muscle circumference (MAMC) in the malnourished group was significantly lower compared to the well-nourished group (21.3 ± 2.2 cm and 24 ± 2.9 cm, respectively. Out of 161 patients with MAMC ≤ 22 cm, 126 (78.3%) were malnourished, and 35 (21.7%) were well-nourished; p-value < 0.001. This was similar to the findings observed by Lawson J. A. et al. [1] and Jai Prakash et al. [5] The body mass index (BMI) in the malnourished group was significantly lower (19.8 ± 1.5 versus 22.5 ± 1.4 kg/m2). These observations were similar to the study done by Jai Prakash et al. [5], Lawson J. A. et al. [1], and R. H. Mak et al. [23]. Out of 141 well-nourished patients, 137 (97.2%) had BMI > 20 kg/m2. This suggests high specificity of BM1 > 20 kg/m2 in ruling out malnutrition. There was a statistically significant direct correlation between TSFT, MAMC, BMI, and eGFR. Median eGFR in the malnourished group was significantly lower than well-nourished group (11.9 ml/min/1.73 m2 versus 24.2 ml/min/1.73m2; p-value < 0.001). In our study, 190//213 (89.2%) patients with malnutrition had eGFR < 30 ml/min/1.73m2; p-value < 0.001. Similar results were reported by Zimmerman J. et al. [24]. This reveals that the incidence of malnourishment increases with deteriorating renal function. Serum albumin in the malnourished and well-nourished groups was 3.03 ± 0.64 mg/dl and 3.50 ± 0.56 mg/dl, respectively; p-value < 0. 001. Similar findings were reported by Emmanuel I. Agaba et al. [25] and by Jai Prakash et al. [5]. Pre-dialysis hypoalbuminemia has also been found to be a strong predictor of mortality in CKD patients undergoing maintenance dialysis [8, 26,27,28]. The low level of serum albumin is also reported as a marker of malnutrition [25, 29, 30]. Hemoglobin in the malnourished group was significantly low compared to the well-nourished group (7.74 ± 0.95 mg/dl and 8.84 ± 0.95mg/dl, respectively; p-value < 0.001). Similar trends were observed by the studies of Emmanuel I. Agaba et al. [25] and Lowrie E. G. et al. [26]. In our study, serum triglyceride (TG) was the only significant parameter found to be increased in malnourished CKD patients as compared to the well-nourished group (149.59 ± 15.28 vs 138. ± 17.23 mg/dl, p < 0.001). Similar observations were made by Emmanuel I. et al. [25] and Jai Prakash et al. [5]. The presence of increased serum TG levels further heightens the already increased cardiovascular risk in malnourished CKD patients. In our study, serum cholesterol was not statistically different between the malnourished and the well-nourished group. In the subgroup analysis of patients with eGFR ≤ 30 ml/min/1.73 m2 and > 30 ml/min/1.73m2, the anthropometric indices in the form of TST, MAMC, and BMI and the laboratory indices in the form of hemoglobin, total protein, and albumin were significantly lower in the malnourished group in comparison with the well-nourished group.
Renal failure contributes to inflammation as a result of the accumulation of pro-inflammatory compounds and a decrease in the activity of superoxide dismutase, and glutathione peroxidase leading to increased oxidative stress and decreased antioxidative activity [31]. High sensitivity CRP was elevated (> 0.6 mg/dl) in 114/213 (53.5%) patients with malnutrition and 53/141 (37.6%) of well-nourished patients; p-value 0.003. Similar observations were made in the study by Jai Prakash et al. [5] in which 83/131 (63%) malnourished patients had elevated CRP compared to 24/72 (33%) in the well-nourished group. Serum ferritin level was 246.77 ± 18.24 mg/L and 237.23 ± 16.13 mg/L, respectively, in the malnourished and well-nourished group, p-value < 0.001. Similar observations were made by Jai Prakash et al. [5]. Serum ferritin and CRP are the markers of inflammation and are significantly elevated in malnourished CKD patients. Serum albumin is also considered a negative phase reactant, and low serum albumin in CKD is thought to be a marker of inflammation than malnutrition. In patients with eGFR > 30 ml/min/1.73 m2, the difference in serum ferritin between the malnourished and the well-nourished group did not reach the statistical difference. This suggests that ferritin as an inflammatory marker becomes more important with eGFR ≤ 30 ml/min/1.73 m2. However, this needs to be reproduced in a bigger study.
This study has certain limitations. It is a single-center hospital-based study and does not reflect the true prevalence of malnutrition in CKD patients in the community. The majority of patients belonged to CKD stage 5 which might be the reason for the very high prevalence of malnutrition in our study group. The high sensitivity of TSFT ≤ 10 mm and high specificity of BMI ≥ 20 kg/m2 need to be reproduced in a bigger study.
Conclusions
Malnutrition is highly prevalent in the pre-dialysis CKD population. The incidence increases with the decrease in eGFR. TSFT ≤ 10 mm compares favorably with SGA in detecting malnutrition (sensitivity > 90%), and BMI > 20 kg/m2 compares favorably with SGA in ruling out malnutrition (specificity 97%). Malnutrition and inflammation often coexist. Early detection and appropriate management are needed.
Availability of data and materials
Data and material will be available on request.
Abbreviations
- TSFT:
-
Triceps skinfold thickness
- MAMC:
-
Mid-arm muscle circumference
- BMI:
-
Body mass index
- SGA:
-
Subjective global assessment
- CRP:
-
C-reactive protein
- eGFR:
-
Estimated glomerular filtration rate
References
Lawson JA, Lazarus R, Kelly JJ (2001) Prevalence and prognostic significance of malnutrition in chronic renal insufficiency. J Ren Nutr 11(1):16–22
Kadiri ME, Nechba RB, Oualim Z (2011) Factors predicting malnutrition in hemodialysis patients. Saudi J Kidney Dis Transpl 22:695–604
Cianciaruso B, Brunori G, Kopple JD, Traverso G, Panarello G, Enia G et al (1995) Cross-sectional comparison of malnutrition in continuous ambulatory peritoneal dialysis and hemodialysis patients. Am J Kidney Dis 26(3):475–486
Tayyem RF, Mrayyan MT (2008) Assessing the prevalence of malnutrition in chronic kidney disease patients in Jordan. J Ren Nutr 18(2):202–209
Prakash J, Raja R, Mishra RN, Vohra R, Sharma N, Wani IA et al (2007) High prevalence of malnutrition and inflammation in un-dialyzed patients with chronic renal failure in developing countries: a single-center experience from eastern India. Ren Fail 29(7):811–816
Marckmann. (1989) Nutritional status and mortality of patients in regular dialysis therapy. J Intern Med 226:429–432
Stenvinkel P, Heimburger O, Lindholm B et al (2000) Are there two types of malnutrition in chronic renal failure? Evidence for relationships between malnutrition, inflammation, and atherosclerosis (MIA syndrome). Nephrol DialTransplant 15:953–960
Leavey SF, Strawderman RL, Jones CA et al (1998) Simple nutritional indicators as independent predictors of mortality in hemodialysis patients. Am J Kidney Dis 31:997–906
Lowrie EG, Huang WH, Lew NL (1995) Death risk predictors among peritoneal dialysis and hemodialysis patients: a preliminary comparison. Am J Kidney Dis 26:220–228
Avram MM, Kopple JD (2003) Malnutrition inflammation complex syndrome in dialysis patients: causes and consequences. Am J Kidney Dis 42:864–881
Ballmer PE, McNurlan MA, Hulter HN, Anderson SE, Garlick PJ, Krapf R (1995) Chronic metabolic acidosis decreases albumin synthesis and induces negative nitrogen balance in humans. J Clin Invest 95:39–45
Lecker SH, Goldberg AL, Mitch WE (2006) Protein degradation by the ubiquitin-proteasome pathway in normal and disease states. J Am Soc Nephrol 17:1807–1819
Manzano AMC (2001) Hypoalbuminemia in dialysis. Is it a marker for malnutrition or inflammation. Rev De Investig Clin 53:152–158
Detsky AS, McLaughlin JR, Baker JP, Johnston N, Whittaker S, Mendelson RA, Jeejeebhoy KN (1987) What is a subjective global assessment of nutritional status? JPEN J Parenteral Enteral Nutr 11(1):8–13
Desbrow B, Bauer J, Blum C et al (2005) Assessment of nutritional status in hemodialysis patients using patient-generated subjective global assessment. J Ren Nutr 15:211–216
Locatelli F, Fouque D, Heimburger O et al (2002) Nutritional status in dialysis patients: a European consensus. Nephrol Dial Transplant 17:563–572
Cooper BA, Bartlett LH, Aslani A, Allen BJ, Ibels LS, Pollock CA (2002) Validity of subjective global assessment as a nutritional marker in end-stage renal disease. Am J Kidney Dis 40(1):126–132
Enia G, Sicuso C, Alati G et al (1993) Subjective global assessment of nutrition in dialysis patients. Nephrol Dial Transplant 8:1094–1098
Young A, Kopple D, Lindholm B et al (1991) Nutritional assessment of continuous ambulatory peritoneal dialysis patients: an international study. Am J Kidney Dis 17:462–471
Oladele CO, Unuigbe E, Chukwuonye II, Obi EC, Ohagwu KA, Oladele G, Ojogwu LI (2021) Assessment of nutritional status in patients with chronic kidney disease in Nigeria. Saudi J Kidney Dis Transpl 32:445–454
Liman HM, Anteyi EA, Oviasu E (2015) Prevalence of malnutrition in chronic kidney disease: a study of patients in a tertiary hospital in Nigeria. Sahel Med J 18(Suppl S1):8–11
Kalantar-Zadeh K, Ikizler TA, Block G, Avram MM, Kopple JD (2003) Malnutrition-inflammation complex syndrome in dialysis patients: causes and consequences. Am J Kidney Dis 42(5):864–881
Mak RH, Cheung W, Cone RD, Marks DL (2006) Leptin and inflammation-associated cachexia in chronic kidney disease. Kidney Int 69(5):794–797
Zimmermann J, Herrlinger S, Pruy A, Metzger T, Wanner C (1999) Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney Int 55(2):648–658
Agaba EI, Agaba PA (2003) Prevalence of malnutrition in Nigerians with chronic renal failure. Int Urol Nephrol 36:89–93
Lowrie EG, Lew NL (1990) Death risk in hemodialysis patients: the predictive value of commonly measured variables and an evaluation of death rate differences between facilities. Am J Kidney Dis 15(5):458–482
Avram MM, Mittman N, Bonomini L, Chattopadhyay J, Fein P (1995) Markers for survival in dialysis: a seven-year prospective study. Am J Kidney Dis 26(1):209–219
Fung F, Sherrard DJ, Gillen DL et al (2002) Increased risk for cardiovascular mortality among malnourished end-stage renal disease patients. Am J Kidney Dis 40(2):307–314
Kaysen GA (2000) Malnutrition and the acute-phase reaction in dialysis patients-how to measure and how to distinguish. Nephrol Dial Transplant 15(10):1521–1524
Riella MC (2000) Malnutrition in dialysis: malnourishment or uremic inflammatory response? Kidney Int 57(3):1211–1232
Qureshi AR, Alvestrand A, Divino-Filho JC, Gutierrez A, Heimbürger O, Lindholm B, Bergström J (2002) Inflammation, malnutrition, and cardiac disease as predictors of mortality in hemodialysis patients. J Am Soc Nephrol 13(Suppl 1):S28–S36
Acknowledgements
Sincere thanks to the patients who participated in the study.
Funding
The study is not funded.
Author information
Authors and Affiliations
Contributions
RS, HS, NB, and IW contributed to the design and development of the study. RS and NB contributed to data collection. RS, HS, NB, and IW contributed to data analysis and interpretation. RS, HS, NB, and IW participated in the writing of the manuscript. RS and IW participated in the critical review. RS, HS, NB, and IW provided approval for the final manuscript. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study is approved by the institutional ethics committee of the Shri Ram Murti Smarak Institute of Medical Sciences under reference no. SRMSIMS/IEC/2018-19/551 dated 26th September 2018. Since this is an observational study, the consent to participate has been waived off by the ethics committee of the Shri Ram Murti Smarak Institute of Medical Sciences.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sheikh, R.Y., Samoon, H.J., Bhat, N.A. et al. Malnutrition and inflammatory parameters in patients with chronic kidney disease stages 3–5 from northern India. Egypt J Intern Med 34, 70 (2022). https://doi.org/10.1186/s43162-022-00149-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43162-022-00149-1