Abstract
Background
Handgrip strength (HGS) and pinch strengths (PS) are the common measures to evaluate hand function and predict general health. Normative values of HGS, PS, and prediction equations of HGS for healthy young adult women have not been reported yet in Saudi Arabia. The aims of the study were to determine the HGS and PS normative values and develop the prediction equations for the established HGS in a sample of healthy female college students, aged 19–25 years. In this descriptive cross-sectional study, 139 healthy female college students were recruited randomly from King Saud University (KSU). Both HGS and PS in kilograms were measured using a Jamar hand dynamometer and pinch gauge respectively with standard testing protocol and instructions.
Results
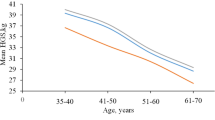
HGS significantly increased with progress in age, while there was no significant effect of age on PS. HGS and PS of the dominant hand were statistically greater than those of the nondominant hand (P < 0.05) in most ages. There were significant correlations between dominant HGS, age (r = 0.7, P < 0.001), palmar width (r = 0.74, P < 0.001), and level of physical activity (r = 0.60, P < 0.001). Regression analysis revealed that palmar width and age were the predictors of dominant HGS and accounted for 55% and 14% of the variation, respectively.
Conclusions
Normative values can be used as a clinical reference in the evaluation of hand function in the rehabilitation process with consideration of age and palmar width for a particular population.
Similar content being viewed by others
Background
The hand is a creative tool that is used for nonverbal communication and an important tactile organ. It implements fine and sensitive tasks. Hands are required for many functions such as grasping, moving, writing, computing, and so many others [1]. Hand gripping represents the complex anatomical and functional structure of the hand [2], which occurred during all the activities of daily living (ADLs). Therefore, the analysis of hand strength, including handgrip strength (HGS) and pinch strengths (PS), is an essential item in upper limb functional evaluation. The gripping movements recommended for functional assessment using dynamometers are HGS, and PS (palmar, key, and tip pinches).
The palmar pinch is used in about 60% of the ADLs. It is a pinch of intermediate strength. It occurs between the thumb, index, and middle finger pulls, such as picking up a pen [2]. It requires the action of index and middle fingers’ superficial flexor muscles and thenar muscles [3] to stabilize of the middle and the proximal phalanges of the thumb in flexion, respectively.
The key pinch is carried out between the thumb pulp and the lateral side of the middle phalange of the index finger, such as putting a key into a lock [2, 3]. The adductor musculature of the thumb performs a significant role in this pinch, which is established by electromyography studies. Tip pinch is the finest and the most precise of the digital pinches and is used for grabbing small objects. It is accomplished by using the thumb and index finger pulps [3, 4].
The evaluation of HGS and PS is very essential in upper limb injury assessment [5]. It helps in clarifying the efficiency of various treatment methods and in assessing the work capability of patients with local hand trauma and injuries or in case of a systematic condition that might affect hand function such as rheumatoid arthritis or muscular dystrophy [5].
HGS is an assessment of the maximum voluntary contraction force of the hand. It is the simplest measuring method of muscle function and a powerful predictor of both future morbidity and mortality rate not only in older population but also in the younger one [6]. HGS and PS are assumed to be a central biomarker of healthy aging [7].
The establishment of normative values of HGS and PS is required in decision-making in hand surgery to evaluate and interpret data, design an accurate treatment program, and evaluate the patients’ capability to return to their jobs [8]. The reported HGS and PS norms are varied significantly in different populations, proposing that universal norms do not exist [9,10,11]. Several studies have established normative values of hand strength using different measurement methods in Indian [12], German [5], Greek [1], Turkish [13, 14], South Korean [15], and Nigerian [16] populations.
Several prognostic factors of hand strength had been reported in the literature, like gender, age, height, weight, body mass index (BMI), wrist and hand circumferences, hand dominance, physical activities, and occupation [14]. Thus, establishing the norms of HGS and PS for each population and even for each geographical region is very crucial for hand therapists to assess impairment and follow progress among patients [17].
Up to the authors’ knowledge, only six studies attempted to estimate the normative values of HGS and PS in the Kingdom of Saudi Arabia (KSA). Four studies were conducted for male children and male adults [17,18,19,20] and two studies for older adult s[21, 22]. However, no studies were conducted yet in healthy young adult women. Therefore, this study aimed to (1) establish the normative values of HGS and PS and (2) develop the prediction equations for the established HGS in a sample of healthy female college students, aged 19–25 years.
Methods
Design and setting
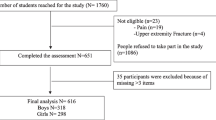
In this descriptive cross-sectional study, a sample of 139 healthy female college students was recruited randomly from King Saud University (KSU), Riyadh, KSA, between March 2018 and November 2019.
Participants
Participants with the following criteria were eligible for the study: (1) healthy Saudi female students and (2) aged 19–25 years old. Exclusion criteria were (1) cardiac, pulmonary, and metabolic disorders affecting muscular strength; (2) trauma, fracture, surgery, and/or deformity of the upper limb and hand; (3) cervical radiculopathy; and (4) presence of pain at the time of evaluation. All participants signed a consent form describing the aims and procedures of the study before enrolling. The research ethics committee at College of Applied Medical Sciences Institutional Review Board, KSU, Riyadh, approved the study with reference number (CAMS 088-3839).
The participants were selected via random sampling using an envelope method. The sample size was calculated using the formula “N > 104 + m”, where N is the sample size and m is the number of independent variables (n = 7). The independent variables were age, height, weight, BMI, wrist circumference, palmar width, and physical activity (PA) level [23]. Therefore, the estimated sample size was 111 participants, and for a possible 10% of dropped out, the sample size increased to 123 participants.
Procedures
The primary investigator examined the integrity of the musculoskeletal and neurofunction of the upper extremities through inspection, palpation, assessment of the active range of motion, and symptoms of pain before the dynamometry measurements.
Demographic and anthropometric measurements
Information regarding the demographic variables such as age, weight, height, BMI, and hand dominance (defined as the ability to use hand to manipulate the ADL such as eating, writing, drawing, and throwing a ball) were collected. Weight and height were measured using calibrated weight (ProMed 6129) with a stadiometer to the nearest kilogram (kg) and centimeter (cm). BMI was calculated as the ratio between the weight and the square of height (kg/m2). The same investigator measured anthropometric hand data using 100-cm non-elastic tape. The circumference of the wrist was measured at the level of the articulation of the radius and the ulna which matches the edge of the tiniest part of the forearm. The palmar width was measured in both hands from the radial side of the metacarpal of the second digit (index finger) and ulnar side of the metacarpal of the fifth digit (small finger). All anthropometric data were measured to the nearest cm, with the forearm and hand rested in a supinated position and the elbow was supported on a table [15, 24].
PA level measurement
The PA level was measured utilizing the short form (7 items) of the international physical activity questionnaire (IPAQ) Arabic version [25]. The metabolic equivalent minutes (MET-min) per week for walking, moderate- and vigorous-intensity activities were calculated as follows: walking, ¼ (3.3 × walking minutes × walking days); moderate activity, ¼ (4.0 × moderate activity minutes × moderate activity days); and vigorous activity, ¼ (8.0 × vigorous activity minutes × vigorous activity days). Furthermore, sufficient vigorous activity was computed based on 3 or more days of vigorous-intensity activity of at least 20 min per day. Likewise, sufficiently moderate and walking activities were computed based on 5 or more days of moderate-intensity and walking of at least 30 min per day. PA levels were classified into three categories: inactive, minimally active, and health-enhancing physically active, according to the scoring system provided by the IPAQ website (www.ipaq.ki.se).
HGS and PS assessment
Valid and reliable Jamar hand dynamometer and a pinch gauge ((749805)-JLW instruments) were used to measure the HGS and PS respectively for both dominant and nondominant hands.
Data collection was performed according to the American Society of Hand Therapists (ASHT) guidelines [26]. The participants were seated on a chair without armrests with their feet flat on the floor, shoulder was adducted and neutrally rotated, elbow flexed at 90°, forearm in a neutral position, and wrist between 0 and 30° extension and between 0 and 15° ulnar deviation. The dynamometer was set at the second handle position. The primary investigator showed all testing positions and provided oral instructions. The participants received verbal encouragement “squeeze the gauge as hard as possible” to exert their maximal force during each trial and hold for 5 s. Each test was performed three times to collect HGS and PS data, and the average was calculated and expressed in kilograms. If a measurement displayed a difference of over 10% from the previously achieved measurements, it would lead to performing a fourth trial [26, 27]. The measurements of HGS and PS were done in alternating order between the dominant and nondominant hands, and a 1-min rest between them to minimize fatigue effect [1, 28]. The calibration of Jamar hand dynamometer and a pinch gauge was tested periodically during the study [28].
Statistical analysis
The distribution of data was examined prior to analysis using Kolmogorov-Smirnov test. The data were presented as a mean ± standard deviation (SD) for continuous variables and as frequency and percentage for noncontinuousvariables. The HGS and PS were collected and stratified by age (in 1-year increments) to capture normative values. The analysis of covariance (ANCOVAs) was utilized to determine the effect of age on HGS and PS. Post hoc analysis (Scheffe test) was utilized to detect the difference in each pair-wise condition. An independent sample t-test was conducted to compare hand strength for the dominant and nondominant hands. Pearson and Spearman correlation coefficients applied to investigate the correlation between HGS and PS as dependent variables and independent variables (demographic, anthropometric, and PA level). Stepwise multiple linear regression model was operated to determine if any of the independent variables predict dominant HGS. The presence of multicollinearity between independent variables was tested using variance inflation factors (VIF) at a cutoff point of 10 [29]. All analyses were performed using SPSS, version 22.0 (SPSS Inc., Chicago, IL, USA) for Windows®. Statistical significance was considered at a P value of < 0.05.
Results
Demographic and anthropometric characteristics of the participants
One hundred eighty-eight participants were evaluated. Forty-nine were excluded due to neurological disorders (n = 20), non-Saudi (n = 15), and did not complete all measurements (n = 14), while 139 participants were eligible and completed the assessment procedures.
Table 1 summarizes the demographic and anthropometric characteristics of the study participants. The mean of age was 21.39 ± 1.68 years (ranged 19–25), and the means of weight, height, and BMI were 60.3 ± 13.4 kg, 158.6 ± 6.2 cm, and 23.81 ± 5.0 kg/cm2 respectively. Overall, there were no significant differences regarding weight, height, BMI, and wrist circumference (of both dominant and nondominant hands) across different ages (P ˃ 0.05 for all). The significant effect of age was reported on palmar width of the dominant hand only (F(6) = 11.41, P < 0.001)—the greater the age, the wider the palmar width. Besides, there were no significant differences between the hands for wrist circumference and palmar width in all age groups (P ˃ 0.05). Right hand dominant was reported in 90.6% of participants, while left-handed found in 9.4%.The majority of the participants 64.8% reported moderate to a high level of physical activity while 35.2% were inactive with a significant difference between age groups (P ˂ 0.05) in favor of older age.
Handgrip strength
Table 2 lists the normative values of HGS and PS including means and SDs, for dominant and nondominant hands. The HGS showed incremental and significant increase as age progresses for both dominant and nondominant hands (F(6) = 24.52, P < 0.001) and (F(6) = 7.19, P < 0.001) respectively. Regarding the dominant hand, significant differences were noted between ages except for 19–21-, 22–24-, and 24–25-year-old women (P > 0.05). Meanwhile, significant differences were reported only between 19 and 22–24 years and between 20- and 23–24-year-old women in the nondominant hand (P > 0.05) in favor to an older age. Overall, dominant HGS was significantly stronger than that of the nondominant (P < 0.05) in all age groups except for 21-year-old women (P > 0.05). The lowest mean difference between dominant and nondominant hands (2.30 kg) was reported in 21 years while the greatest mean difference (6.07 kg) was observed in 25-year-old women.
Pinch strengths
No differences were reported in PS across age groups for both dominant and nondominant hands (F(6) = 1.85, P > 0.05 vs (F(6) = 1.43, P > 0.05), (F(6) = 1.49, P > 0.05 vs F(6) = 1.13, P > 0.05), and (F(6) = 0.75, P > 0.05) vs F(6) = 1.91, P > 0.05) for palmar, key, and tip pinch respectively. However, the dominant hand exhibited greater PS than that of the nondominant hand (P < 0.0001) in all age groups except for 19–21 years (P > 0.05) for palmar pinch strength, 19 and 21 years for key pinch (P > 0.05), and 19, 21, and 25years for tip pinch (P > 0.05). For three types of pinch strengths, palmar pinch was the strongest followed by key pinch and tip pinch. The maximum values were observed at 25-year-old women for both hands.
Table 3 demonstrates the correlationsof HGS and PS of the dominant hand with the independent variables in addition to their correlation to each other. HGS was positively and strongly correlated with age (r = 0.71, P < 0.05) and palmar width (r = 0.74, P < 0.05) and moderately correlated with PA level (r = 0.603, P < 0.05). Furthermore, HGS demonstrated moderate to weak significant correlations with a palmar pinch, key pinch, and tip pinch (r = 0.455, r = 0.283, and r = 0.184, all P < 0.05) respectively. Palmar pinch of the dominant hand showed positive weak correlations with age and PA level and moderate correlation with palmar width respectively (P < 0.05). On the other hand, there was a weak positive correlation between both key and tip pinches with palmar width respectively and weak correlation of key pinch with PA level (P < 0.05).
A stepwise multiple linear regression analysis revealed that palmar width and age were the predictors of HGS of the dominant hand whereby 55% (P˂0.001) and 14% (P˂ 0.001) of the variation in HGS could be explained by palmar width and age respectively (Table 4). Prediction equations of HGS include:
Discussion
This study aimed to establish the HGS and PS normative values and develop the prediction equations for the established HGS in a sample of healthy female college students, aged 19–25 years. The results illustrated that HGS incrementally increases with the age progress from 19 to 25 years old. HGS and PS of the dominant hand are stronger than those of the nondominant hand, and the variability in dominant HGS can be explained by palmar width (55%) and age (14%). The established prediction equations using these variables could be applied easily in both rehabilitation and research settings.
The main problem when comparing normative values among similar studies starts from variations in the methodology, data presentation [29], and differences in sample size from previous studies [30]. The mean of HGS was observed to be 28.82 kg and 25.21 kg for dominant and nondominant hands, respectively. This finding agrees with the results of the studies conducted in the same age groups in other countries such as Germany [5], Turkey [13], Brazil [31], and Iran [9]. However, other studies showed lower values of HGS such as South Korea [15], Sri Lanka [32], India [11], and Nigeria [16].
The PS was reported in different studies. In line with the value estimated in the current study (6.2 ± 1.9 for the dominant hand), Dianat et al. [33] found that palmar pinch was 6 kg in 21–25-year-old women. In contrast, Mathiowetz et al. [28] reported a higher value of palmar pinch strength. Meanwhile, the values of key pinch reported in the current study were 5.56 ± 1.0 and 4.2 ± 0.9 kg for dominant and nondominant hands, respectively, which were lower than those reported in previous studies [28, 33, 34]. Regarding tip pinch, the value accounted for in this study was 4.7 ± 1.5 kg for the dominant hand which is supported by Dianat et al. (4.5 kg) [33]. In contrast, many studies showed higher values of tip pinch for both dominant and nondominant hands [9, 30]. According to Han et al., the key pinch was the strongest followed by tip pinch and tripod pinch [35]; however, our study showed that palmar pinch is stronger than key pinch and tip pinch.
The discrepancies of the HGS and PS between participants in different studies could be attributed to research type, geographic differences, genetic elements, nutritional deficits, and social/cultural contradictions [36]. The anthropometric difference among different populations might give another explanation for HGS and PS variation among studies [16]. When studying HGS and PS, it is important to recognize their association with the anthropometric measures as they are the indicators of nutritional status. Likewise, this study investigated the correlation between hand strength (HGS and PS), demographic variables (height, weight, BMI, and PA level), and hand anthropometric measurements (wrist circumference and palmar width) in the dominant hand.
The findings of this study show that dominant HGS and palmar pinch strength positively correlates with age, palmar width, and PA level. These results agree with Amaral et al. [37] and Nevill and Holder [38]. However, Nakandala et al. reported positive correlations between both dominant and nondominant HGS, weight, and BMI and in adult women [32]. In addition, weak positive correlations were found between palmar width, key pinch, and tip pinch. The current study confirmed that there were positive correlations between HGS and PS (palmar, key, and tip pinch) which were supported by Han et al. [35].
The results reported that palmar width and age are the predictors of HGS. However, Lim et al. reported that occupation, weight, and height are the predictors of HGS [39]. In addition, Macdermid et al. stated that height was the predictor of HGS which was not consistent with the current findings [40].
Palmar width was the strongest factor associated with HGS and the main predictor in the regression equation in our study. The positive influence of palmar width on HGS was consistent with previous studies [32, 40]. This can be related to the fact that larger palmar width shows larger muscle mass [41] and bone which reflected on grip strength. Age was considered the second predictor of HGS and explained 14% of the total variance. This agreed with previous studies [32, 40] and disagreed with Angst et al. where they concluded that weight is one of the HGS predictors [34]. There was no predictor variable with a VIF value of 10 or more which shows the absence of multicollinearity between the predictor variables [29]. In our study, both the palmar width and age showed a significant contribution to HGS in regression analysis and the VIF was 1.40 and 1.38 for palmar width and age, respectively.
The study has several limitations; first, it was performed only in one province in Saudi Arabia. The results cannot estimate the values of the residents in other areas of Saudi Arabia, or other ethnicities; second, despite calculation of sample size, a larger sample size is recommended for future studies; third, incorporation of adult women aged 19–25 years.
Conclusion
The current study presents specific norms for HGS and PS in Saudi young adult women by age and dominance which can be used as a referencevalue during patient’s rehabilitation with the same age group.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ADLs:
-
Activities of daily living
- ANCOVAs:
-
The analysis of covariance
- BMI:
-
Body mass index
- HGS:
-
Handgrip strength
- KSA:
-
Kingdum of Saudi Arabia
- PA:
-
Physical activity
- PS:
-
Pinch strengths
- SD:
-
Standard deviation
- VIF:
-
Variance inflation factor
References
Mitsionis G, Pakos EE, Papakostas T, Beris AE. Normative data on hand grip strength in a Greek adult population. Int Orthop. 2009:713–7. https://doi.org/10.1007/s00264-008-0551-x.
Ferreira ACC, Shimano AC, Mazzer N, Barbieri CH, Elui VMC, Fonseca MCR. Grip and pinch strength in healthy children and adolescents. Acta Ortop Bras. 2011;19(2):92–7. https://doi.org/10.1590/S1413-8522011000200006.
Klein LJ. Evaluation of the hand and upper extremity. In: Fundamentals of hand therapy e-book: clinical reasoning and treatment guidelines for common diagnoses of the upper extremity, vol. 12. St. Louis: Elsevier; 2014. p. 67–86.
Aulicino PL. Clinical examination of the hand. In: Hunter JM, Mackin EJ, Callahan AD, Skirven TM, Schneider LH, Osterman AL, editors. Rehabilitation of the hand and upper extremity. 5th ed. St. Louis: Mosby; 2002. p. 120–42.
Steiber N. Strong or weak handgrip? Normative reference values for the German population across the life course stratified by sex, age, and body height. PLoS One. 2016;11(10):e0163917. https://doi.org/10.1371/journal.pone.0163917.
Ensrud KE, Ewing SK, Taylor BC, Fink HA, Cawthon PM, Stone KL, Hillier TA, Cauley JA, Hochberg MC, Rodondi N, Tracy JK, Cummings SR. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Intern Med. 2008;168(4):382–9. https://doi.org/10.1001/archinternmed.2007.113.
Martin-ruiz C, Zglinicki TV. Conference on ‘Nutrition and healthy ageing’ Symposium 1: Biology of ageing biomarkers of healthy ageing: expectations and validation. In: Proceedings of the Nutrition Society Proceedings of the Nutrition Society; 2017. p. 422–9.
Mathiowetz V, Weber K, Volland G, Kashman N. Reliability and validity of grip and pinch strength evaluations. J Hand Surg [Am]. 1984;9:222–6.
Mohammadian M, Choobineh A, Haghdoost A. Normative data of grip and pinch strengths in healthy adults of Iranian population. Iran J Public Health. 2014;43(8):1113–22.
Oksuzyan A, Demakakos P, Shkolnikova M, Thinggaard M, Vaupel JW, Christensen K, Shkolnikov VM. Handgrip strength and its prognostic value for mortality in Moscow, Denmark, and England. PLoS One. 2017;12(9). https://doi.org/10.1371/journal.pone.0182684.
Cooper R, Benzeval M, Deary IJ, Dennison EM, Der G, Gale CR, Inskip HM, Jagger C, Kirkwood TB, Lawlor DA, Robinson SM. Grip strength across the life course: normative data from twelve British studies; 2014. p. A4–5. https://doi.org/10.1371/journal.pone.0113637.
Mullerpatan RP, Karnik G, John R. Grip and pinch strength: normative data for healthy Indian adults. Hand Therapy. 2013;18(1):11–6. https://doi.org/10.1177/2F1758998313479874.
Haynes E, Debeliso M. The relationship between CrossFit performance and grip strength. Turk J Kinesiol. 2019;5(1):15–21. https://doi.org/10.31459/turkjkin.515874.
Ekşioğlu M. Normative static grip strength of population of Turkey, effects of various factors and a comparison with international norms. Appl Ergon. 2016;52:8–17. https://doi.org/10.1016/j.apergo.2015.06.023.
Kim CR, Jeon YJ, Kim MC, Jeong T, Koo WR. Reference values for hand grip strength in the South Korean population. PLoS One. 2018;13(4):e0195485. https://doi.org/10.1371/2Fjournal.pone.0195485.
Adedoyin RA, Ogundapo FA, Mbada CE, Adekanla BA, Johnson OE, Onigbinde TA, Emechete AA. Reference values for handgrip strength among healthy adults in Nigeria. Hong Kong Physiother J. 2009;27(1):21–9. https://doi.org/10.1016/S1013-7025(10)70005-1.
Omar MT, Alghadir AH, Zafar H, Al Baker S. Hand grip strength and dexterity function in children aged 6-12 years: a cross-sectional study. J Hand Ther. 2018;31(1):93–101. https://doi.org/10.1016/j.jht.2017.02.004.
Omar MT, Alghadir A, Al Baker S. Norms for hand grip strength in children aged 6–12 years in Saudi Arabia. Dev Neurorehabil. 2015;18(1):59–64. https://doi.org/10.3109/17518423.2014.967878.
Alahmari KA, Kakaraparthi VN, Reddy RS, Silvian PS, Ahmad I, Rengaramanujam K. Percentage difference of hand dimensions and their correlation with hand grip and pinch strength among schoolchildren in Saudi Arabia. Niger J Clin Pract. 2019;22(10):1356–64. https://doi.org/10.4103/njcp.njcp_121_19.
Alahmari KA, Silvian SP, Reddy RS, Kakaraparthi VN, Ahmad I, Alam MM. Hand grip strength determination for healthy males in Saudi Arabia: a study of the relationship with age, body mass index, hand length and forearm circumference using a hand-held dynamometer. J Int Med Res. 2017;45(2):540–8. https://doi.org/10.1177/0300060516688976.
Alqahtani B, Alenazi A, Alshehri M, Alqahtani M, Elnaggar R. Reference values and associated factors of hand grip strength in elderly Saudi population: a cross-sectional study. BMC Geriatr. 2019;19(1):271. https://doi.org/10.1186/s12877-019-1288-7.
Bindawas SM, Vennu V, Al-Orf SM, Alshammari SA, Al-Amoud MM, Calder PC, Al-Muammar MN, Alhamdan AA. Normative data for handgrip strength in Saudi older adults visiting primary health care centers. Medicina. 2019;55(6):1–9. https://doi.org/10.3390/2Fmedicina55060251.
Green SB. How many subjects does it take to do a regression analysis? Multivar Behav Res. 1991;26:499–510. https://doi.org/10.1207/s15327906mbr2603_7.
Li K, Hewson DJ, Duchene J, Hogrel JY. Predicting maximal grip strength using hand circumference. Man Ther. 2010;15(6):579–85. https://doi.org/10.1016/j.math.2010.06.010.
Helou K, El Helou N, Mahfouz M, Mahfouz Y, Salameh P, Harmouche-Karaki M. Validity and reliability of an adapted arabic version of the long international physical activity questionnaire. BMC Public Health. 2018;18(1):1–8.
MacDermid J, Solomon G, Valdes K. Clinical assessment recommendations. American Society of Hand Therapists; 2015.
Lam NW, Goh HT, Kamaruzzaman SB, Chin AV, Poi PJ, Tan MP. Normative data for hand grip strength and key pinch strength, stratified by age and gender for a multiethnic Asian population. Singapore Med J. 2016;57(10):578–84. https://doi.org/10.11622/smedj.2015164.
Mathiowetz V, Kashman N, Volland G, Weber K, Dowe M, Rogers S. Grip and pinch strength: normative data for adults. Arch Phys Med Rehabil. 1985;66(2):69–74.
Craney TA, Surles JG. Model-dependent variance inflation factor cutoff values. Quality Engineering. 2002;14(3):391–403. https://doi.org/10.1081/QEN-120001878.
Ugurlu Ü, Özdogan H. Age- and gender-specific normative data of pinch strengths in a healthy Turkish population. J Hand Surg Eur Vol. 2011;37(5):436–46. https://doi.org/10.1177/1753193411428270.
Schlüssel MM, dos Anjos LA, de Vasconcellos MT, Kac G. Reference values of handgrip dynamometry of healthy adults: a population-based study. Clin Nutr. 2008;27(4):601–7.
Nakandala P, Manchanayake J, Narampanawa J, Neeraja T, Pavithra S, Mafahir M, Dissanayake J. Descriptive study of hand grip strength and factors associated with it in a group of young undergraduate students in university of Peradeniya, Sri Lanka who are not participating in regular physical training. Int J Physiother. 2019;6(3):82–8. https://doi.org/10.15621/ijphy/2019/v6i3/183876.
Dianat I, Feizi H, Hasan-khali K. Pinch strengths in healthy Iranian children and young adult population. Health Promot Perspect. 2015;5(1):52–8. https://doi.org/10.15171/hpp.2015.007.
Angst F, Drerup S, Werle S, Herren DB, Simmen BR, Goldhahn J. Prediction of grip and key pinch strength in 978 healthy subjects. BMC Musculoskelet Disord. 2010;11(1):2–7. https://doi.org/10.1186/2F1471-2474-11-94.
Han SH, Nam KS, Cho YS, Ryu KJ. Normative data on hand grip strength. Nov Physiother. 2011;1(1):1–4. https://doi.org/10.4172/2165-7025.1000102.
Jeune B, Skytthe A, Cournil A, Greco V, Gampe J, Berardelli M, Andersen-Ranberg K, Passarino G, De Benedictis G, Robine JM. Handgrip strength among nonagenarians and centenarians in three European regions. J Gerontol Ser A Biol Sci Med Sci. 2006;61(7):707–12. https://doi.org/10.1093/gerona/61.7.707.
Amaral CA, Amaral TL, Monteiro GT, Vasconcellos MT, Portela MC. Hand grip strength: reference values for adults and elderly people of Rio Branco, Acre, Brazil. PLoS One. 2019;14(1):e0211452. https://doi.org/10.1371/journal.pone.0211452.
Nevill AM, Holder HR. Modelling handgrip strength in the presence of confounding variables: results from the Allied Dunbar National Fitness Survey. Ergonomics. 2000;43(10):1547–58. https://doi.org/10.1080/001401300750003970.
Lim SH, Kim YH, Lee JS. Normative data on grip strength in a population-based study with adjusting confounding factors: Sixth Korea national health and nutrition examination survey (2014 – 2015). Int J Environ Res Public Health. 2019;16(12):2235. https://doi.org/10.3390/2Fijerph16122235.
Macdermid JC, Fehr LB, Lindsay KC. The effect of physical factors on grip strength and dexterity. Br J Hand Ther. 2002;7(4):112–8. https://doi.org/10.1177/2F175899830200700401.
Ramírez Vélez R, Correa Bautista JE, García Hermoso A, Cano CA, Izquierdo M. Reference values for handgrip strength and their association with intrinsic capacity domains among older adults. J Cachexia Sarcopenia Muscle. 2019;10(2):278–86. https://doi.org/10.1002/jcsm.12373.
Acknowledgements
N/A
Authors' contributions
AS suggested the research idea, collected the data, analyzed and interpreted the data regarding the participants’ demographics characteristics, and performed measurement of handgrip and pinch strengths. MO and OA revised the data analysis and they were contributors in the writing process. All authors read and approved the final version of the manuscript.
Funding
The authors confirm that there is no financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the College of Applied Medical Sciences Institutional Review Board, King Saud University, with reference number (CAMS 088-3839). All the participants signed a written informed consent before starting the study.
Consent for publication
N/A
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shaheen, A.A.M., Omar, M.T.A. & Ali, O.I. Normative values of handgrip and pinch strengths in healthy female college students in Riyadh, Saudi Arabia: a cross-sectional study. Bull Fac Phys Ther 26, 1 (2021). https://doi.org/10.1186/s43161-021-00019-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43161-021-00019-x